Abstract
Purpose
Simvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, has antioxidant and anti-inflammatory properties that are independent of lipid-lowering abilities. This experiment was carried out to explore the effects of simvastatin on apoptosis in vulnerable atherosclerotic plaques of apoE-deficient mice.
Methods and results
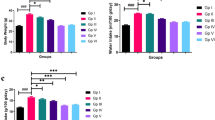
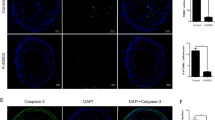
Eight weeks-old apoE−/− mice were fed a Western-type diet. Vulnerable atherosclerotic lesions were formed in the branchiocephalic artery at the age of 30-weeks, before simvastatin administration for 8 weeks. Simvastatin did neither affect the levels of plasma glucose and lipids, nor the size of atherosclerotic lesions. Analysis of plaque composition showed that simvastatin decreased the area of lipid core and increased the amounts of macrophages and smooth muscle cells in atherosclerotic plaques of apoE−/− mice. In addition, simvastatin down-regulated the expression of vascular cell adhesion molecule-1 (VCAM-1) by both inhibition of nuclear factor kappa B (NF-кB) activation and suppression of the expression of the receptor for advanced glycation end products (RAGE). Moreover, we found that simvastatin administration led to reduced TUNEL-positive cells in the aortic root lesions, accompanied by up-regulation of Bcl-2 and Bcl-xL expression, and decreased P53 expression as shown by Western blot.
Conclusion
In the present study, we show novel data to suggest that simvastatin could suppress apoptosis in vulnerable atherosclerotic plaques of apoE−/− mice by regulating the expression of apoptosis-related proteins, such as p53, Bcl-2 and Bcl-xL.




Similar content being viewed by others
References
Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303.
Thorp E, Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol. 2009;86:1089–95.
Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 2009;50:S382–387.
Ihling C, Menzel G, Wellens E, Mönting JS, Schaefer HE, Zeiher AM. Topographical association between the cyclin-dependent kinases inhibitor P21, p53 accumulation, and cellular proliferation in human atherosclerotic tissue. Arterioscler Thromb Vasc Biol. 1997;17:2218–24.
von der Thüsen JH, van Vlijmen BJ, Hoeben RC, et al. Induction of atherosclerotic plaque rupture in apolipoprotein E−/− mice after adenovirus-mediated transfer of p53. Circulation. 2002;105:2064–70.
Tabas I. p53 and atherosclerosis. Circ Res. 2001;88:747–9.
Thorp E, Li Y, Bao L, et al. Brief report: increased apoptosis in advanced atherosclerotic lesions of Apo E−/− mice lacking macrophage Bcl-2. Arterioscler Thromb Vasc Biol. 2009;29:169–72.
Gautier EL, Huby T, Witztum JL, et al. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–804.
Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–9.
Wierzbicki AS, Poston R, Ferro A. The lipid and non-lipid effects of statins. Pharmacol Ther. 2003;99:95–112.
Marzilli M. Pleiotropic effects of statins: evidence for benefits beyond LDL-cholesterol lowering. Am J Cardiovasc Drugs. 2010;10:S3–9.
Zuckerman SH, Evans GF, Schelm JA, Eacho PI, Sandusky G. Estrogen-mediated increases in LDL cholesterol and foam cell-containing lesions in human ApoB100xCETP transgenic mice. Arterioscler Thromb Vasc Biol. 1999;19:1476–83.
Bea F, Blessing E, Bennett B, Levitz M, Wallace EP, Rosenfeld ME. Simvastatin promotes atherosclerotic plaque stability in apoE-deficient mice independently of lipid lowering. Arterioscler Thromb Vasc Biol. 2002;22:1832–7.
Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–8.
Tekabe Y, Li Q, Rosario R, et al. Development of receptor for advanced glycation end products-directed imaging of atherosclerotic plaque in a murine model of spontaneous atherosclerosis. Circ Cardiovasc Imaging. 2008;1:212–9.
Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605.
Bierhaus A, Schiekofer S, Schwaninger M, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-кB. Diabetes. 2001;50:2792–808.
Basta G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis. 2008;196:9–21.
Sun L, Ishida T, Yasuda T, et al. RAGE mediates oxidized LDL-induced pro-inflammatory effects and atherosclerosis in non-diabetic LDL receptor-deficient mice. Cardiovasc Res. 2009;82:371–81.
Harja E, Bu DX, Hudson BI, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–94.
Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007;219:187–203.
Merched AJ, Williams E, Chan L. Macrophage-specific p53 expression plays a crucial role in atherosclerosis development and plaque remodeling. Arterioscler Thromb Vasc Biol. 2003;23:1608–14.
Okada S, Zhang H, Hatano M, Tokuhisa T. A physiologic role of Bcl-xL induced in activated macrophages. J Immunol. 1998;160:2590–6.
Chatterjee D, Han Z, Mendoza J, et al. Monocytic differentiation of HL-60 promyelocytic leukemia cells correlates with the induction of Bcl-xL. Cell Growth Differ. 1997;8:1083–9.
Antignani A, Youle RJ. The cytokine, granulocyte-macrophage colony-stimulating factor (GM-CSF), can deliver Bcl-XL as an extracellular fusion protein to protect cells from apoptosis and retain differentiation induction. J Biol Chem. 2007;282:11246–54.
Funding
This work was supported by grants from National Basic Research Program of China (2012CB517502) and National Natural Science Foundation of China (81070634).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Weiwei Qin, Yonggang Lu, and Chengyan Zhan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Qin, W., Lu, Y., Zhan, C. et al. Simvastatin Suppresses Apoptosis in Vulnerable Atherosclerotic Plaques Through Regulating the Expression of p53, Bcl-2 and Bcl-xL. Cardiovasc Drugs Ther 26, 23–30 (2012). https://doi.org/10.1007/s10557-011-6347-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-011-6347-z