Abstract
Introduction
We investigated the possible protective effect of poly (ADP-ribose) polymerase (PARP) inhibition in preventing endothelial dysfunction induced by hyperhomocysteinemia (Hhcy).
Methods
Sprague–Dawley rats were divided into Hhcy group, Hhcy + 3-aminobenzamide(3-AB) group, control group and control + 3-AB group. A high-methionine diet was given to induce hyperhomocysteinemia. In Hhcy + 3-AB and control + 3-AB groups, rats were injected intraperitoneally with 3-AB (inhibitor of PARP). After 45 days, ultrastructural changes of aortas were observed by transmission electron microscope. Vascular reactivity of thoracic aortic rings was measured in organ chambers. PARP activity was detected. The levels of plasma total homocysteine, nitrite/nitrate, endothelin (ET)-1 and malondialdehyde were assayed.
Results
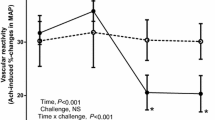
Rats in Hhcy group developed severe hyperhomocysteinemia and significant loss of endothelial function as measured by both vascular rings and levels of nitrite/nitrate and ET-1. Malondialdehyde levels increased significantly in Hhcy rats compared with control rats. 3-AB improved Ach-induced, NO-mediated vascular relaxation and stabilized the level of nitrite/nitrate and ET-1. Obvious improvement of ultrastructure can be observed in Hhcy + 3-AB group.
Conclusions
These results suggest that pharmacological inhibition of PARP prevents the development of endothelial dysfunction in rats with hyperhomocysteinemia which may represent a novel approach to improve vascular dysfunction associated with hyperhomocysteinemia.



Similar content being viewed by others

References
Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338:1042–50.
McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–28.
Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801–9.
Harker LA, Ross R, Slichter SJ, Scott CR. Homocysteine-induced arteriosclerosis: the role of endothelial cell injury and platelet response in its genesis. J Clin Invest. 1976;58:731–41.
Jagtap P, Szabó C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–40.
Pacher P, Szabó C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular disease: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–60.
Liaudet L, Soriano FG, Szabó E, Viráq L, Mabley JG, Salzman AL, et al. Protection against hemorrhagic shock in mice genetically deficient in poly (ADP-ribose) polymerase. Proc Natl Acad Sci USA. 2000;97:10203–8.
Zingarelli B, Salzman AL, Szabo C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intracellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ Res. 1998;83:85–94.
Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabó É, Szabó C. The role of poly (ADP-ribose) polymerase in the development of cardiovascular dysfunction in diabetes mellitus. Diabetes 2002;51:514–21.
Li M, Chen J, Li YS, Feng YB, Zeng QT. Folic acid reduces chemokine MCP-1 release and expression in rats with hyperhomocystinemia. Cardiovasc Pathol. 2007;16:305–9.
Ungvari Z, Pacher P, Rischák K, Szollár L, Koller A. Dysfunction of nitric oxide mediation in isolated rat arterioles with methionine diet-induced hyperhomocysteinemia. Arterioscler Thromb Vasc Biol. 1999;19:1899–904.
Okhawa H, Ohishi N. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8.
Schulz E, Anter E, Keaney JF. Oxidative stress, antioxidants, and endothelial function. Curr Med Chem. 2004;11:1093–104.
Dayal S, Bottiglieri T, Arning E, Maeda N, Malinow MR, Sigmund CD, et al. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circ Res. 2001;88:1203–9.
Cohen R, Loscalzo J. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemiam. J Clin Invest. 2000;106:483–91.
Tasatargil A, Dalaklioglu S, Sadan G. Poly(ADP-ribose) polymerase inhibition prevents homocsyteine-induced endothelial dysfunction in the isolated rat aorta. Pharmacology 2004;72:99–105.
Starkebaum G, Harlan JM. Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine. J Clin Invest. 1986;77:1370–6.
Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest. 1996;98:5–7.
Upchurch GR, Welch GN, Loscalzo J. Homocysteine, EDRF and endothelial function. J Nutr. 1996;126:1290S–4S.
Ungvari Z, Csiszar A, Bagi Z, Koller A. Impaired nitric oxide-mediated flow-induced coronary dilation in hyperhomocysteinemia: morphological and functional evidence for increased peroxynitrite formation. Am J Pathol. 2002;161:145–53.
Levrand S, Pacher P, Pesse B, Rolli J, Feihl F, Waeber B, et al. Homocysteine induces cell death in H9C2 cardiomyocytes through the generation of peroxynitrite. Biochem Biophys Res Commun. 2007;359:445–50.
Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424.
Draper H, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–31.
Pacher P, Liaudet L, Bai P, Virag L, Mabley JG, Hasko G, et al. Activation of poly (ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther. 2002;300:862–7.
Radovits T, Seres L, Gero D, Berger I, Szabó C, Karck M, et al. Single dose treatment with PARP-inhibitor INO-1001 improves aging-associated cardiac and vascular dysfunction. Exp Gerontol. 2007;42:676–85.
Radovits T, Lin LN, Zotkina J, Gero D, Szabó C, Karck M, et al. Poly(ADP-ribose) polymerase inhibition improves endothelial dysfunction induced by reactive oxidant hydrogen peroxide in vitro. Eur J Pharmacol. 2007;564:158–66.
Tangurek B, Ozer N, Sayar N, Terzi S, Yilmaz H, Dayi SU, et al. The relationship between endothelial nitric oxide synthase gene polymorphism (T-786C) and coronary artery disease in the Turkish population. Heart Vessels. 2006;21:285–90.
Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjati K, Szabó C. Endothelial dysfunction in aging animals: the role of poly (ADP-ribose) polymerase activation. Br J Pharmacol. 2002;135:1347–50.
Soriano FG, Pacher P, Mabley J, Liaudet L, Szabó C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly (ADP-ribose) polymerase. Circ Res. 2001;89:684–91.
Zingarelli B, Cuzzocrea S, Zsengeller Z, Salzman AL, Szabó C. Protection against myocardial ischemia and reperfusion injury by 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase. Cardiovasc Res. 1997;36:205–15.
Pacher P, Vaslin A, Benko R, Mabley JG, Liaudet L, Haskó G, et al. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J Pharmacol Exp Ther. 2004;311:485–91.
Radovits T, Lin LN, Zotkina J, Gero D, Szabo C, Karck M, et al. Poly(ADP-ribose) polymerase inhibition improves endothelial dysfunction induced by reactive oxidant hydrogen peroxide in vitro. Eur J Pharmacol. 2007;564:158–66.
Bowes J, McDonald MC, Piper J, Thiemermann C. Inhibitors of poly(ADP-ribose) synthetase protect rat cardiomyocytes against oxidant stress. Cardiovasc Res. 1999;41:126–34.
Acknowledgments
This work was supported by grants from Chenguang plan of Wuhan in China (NO:[2006] 40) and National Basic Research Program of China (973 Program): 2007CB512000;2007CB512005. We thank Professor Liu Changjing in department of physiology of Tongji Medical College for his technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xian Yu and Xiang Cheng contribute to the work equally.
This work was supported by grants from Chenguang plan of Wuhan in China (NO:[2006] 40) and National Basic Research Program of China (973 Program): 2007CB512000;2007CB512005.
Rights and permissions
About this article
Cite this article
Yu, X., Cheng, X., Xie, Jj. et al. Poly (ADP-ribose) Polymerase Inhibition Improves Endothelial Dysfunction Induced by Hyperhomocysteinemia in Rats. Cardiovasc Drugs Ther 23, 121–127 (2009). https://doi.org/10.1007/s10557-008-6146-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-008-6146-3