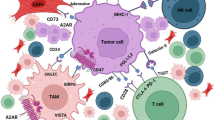
Abstract
Emerging evidence has unveiled a critical role for immunological parameters in predicting tumor prognosis and clinical responses to anticancer therapeutics. On the other hand, responsiveness to anticancer drugs greatly modifies the repertoires, phenotypes, and immunogenicity of tumor-infiltrating immune cells, serving as a critical factor to regulate tumorigenic activities and the emergence of therapy-resistant phenotypes. Tumor-associated immune functions are influenced by distinct or overlapping sets of therapeutic modalities, such as cytotoxic chemotherapy, radiotherapy, or molecular-targeted therapy, and various anticancer modalities have unique properties to influence the mode of cross-talk between tumor cells and immune cells in tumor microenvironments. Thus, it is critical to understand precise molecular machineries whereby each anticancer strategy has a distinct or overlapping role in regulating the dynamism of reciprocal communication between tumor and immune cells in tumor microenvironments. Such an understanding will open new therapeutic opportunities by harnessing the immune system to overcome resistance to conventional anticancer drugs.


Similar content being viewed by others
References
Higgins, C. F. (2007). Multiple molecular mechanisms for multidrug resistance transporters. Nature, 446, 749–757.
Reya, T., Morrison, S. J., Clarke, M. F., & Weissman, I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414, 105–111.
Sharma, S. V., Lee, D. Y., Li, B., Quinlan, M. P., Takahashi, F., Maheswaran, S., et al. (2010). A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell, 141, 69–80.
Holohan, C., Van Schaeybroeck, S., Longley, D. B., & Johnston, P. G. (2013). Cancer drug resistance: an evolving paradigm. Nature Reviews Cancer, 13, 714–726.
Roesch, A., Fukunaga-Kalabis, M., Schmidt, E. C., Zabierowski, S. E., Brafford, P. A., Vultur, A., et al. (2010). A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell, 141, 583–594.
Roesch, A., Vultur, A., Bogeski, I., Wang, H., Zimmermann, K. M., Speicher, D., et al. (2013). Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell, 23, 811–825.
Magee, J. A., Piskounova, E., & Morrison, S. J. (2012). Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell, 21, 283–296.
Clevers, H. (2011). The cancer stem cell: premises, promises and challenges. Nature Medicine, 17, 313–319.
Haber, D. A., Bell, D. W., Sordella, R., Kwak, E. L., Godin-Heymann, N., Sharma, S. V., et al. (2005). Molecular targeted therapy of lung cancer: EGFR mutations and response to EGFR inhibitors. Cold Spring Harbor Symposia on Quantitative Biology, 70, 419–426.
Poulikakos, P. I., & Rosen, N. (2011). Mutant BRAF melanomas—dependence and resistance. Cancer Cell, 19(1), 11–15.
Nahta, R., Yu, D., Hung, M. C., Hortobagyi, G. N., & Esteva, F. J. (2006). Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nature Clinical Practice Oncology, 3(5), 269–280.
Gilbert, L. A., & Hemann, M. T. (2010). DNA damage-mediated induction of a chemoresistant niche. Cell, 143, 355–366.
Pallasch, C. P., Leskov, I., Braun, C. J., Vorholt, D., Drake, A., & Soto-Feliciano, Y. M. (2014). Sensitizing protective tumor microenvironments to antibody-mediated therapy. Cell, 156(3), 590–602.
Farmer, P., Bonnefoi, H., Anderle, P., Cameron, D., Wirapati, P., Becette, V., et al. (2009). A stroma-related signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nature Medicine, 15, 68–74.
Iliopoulos, D., Hirsch, H. A., & Struhl, K. (2010). An epigenetic switch involving NF-kB, Lin28, Let-7 MicroRNA, and IL-6 links inflammation to cell transformation. Cell, 139, 693–706.
Gilvennikov, S. I., Greten, F. R., & Karin, M. (2010). Immunity, inflammation, and cancer. Cell, 140, 883–899.
Lake, R. A., & Robinson, B. W. (2005). Immunotherapy and chemotherapy—a practical partnership. Nature Reviews Cancer, 5(5), 397–405.
Zitvogel, L., Galluzzi, L., Smyth, M. J., & Kroemer, G. (2013). Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity, 39, 74–88.
Jinushi, M., Yagita, H., Yoshiyama, H., & Tahara, H. (2013). Putting the brakes on anticancer therapies: suppression of innate immune pathways by tumor-associated myeloid cells. Trends in Molecular Medicine, 9, 536–545.
Bruchard, M., Mignot, G., Derangère, V., Chalmin, F., Chevriaux, A., Végran, F., et al. (2013). Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nature Medicine, 19(1), 57–64.
Huang, B., Zhao, J., Li, H., He, K. L., Chen, Y., & Chen, S. H. (2005). Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Research, 65(12), 5009–5014.
Huang, B., Zhao, J., Unkeless, J. C., Feng, Z. H., & Xiong, H. (2008). TLR signaling by tumor and immune cells: a double-edged sword. Oncogene, 27(2), 218–224.
Dranoff, G. (2004). Cytokines in cancer pathogenesis and cancer therapy. Nature Reviews Cancer, 4, 11–22.
Lin, W. W., & Karin, M. (2007). A cytokine-mediated link between innate immunity, inflammation, and cancer. Journal of Clinical Investigation, 117, 1175–1183.
Tye, H., Kennedy, C. L., Najdovska, M., McLeod, L., McCormack, W., Hughes, N., et al. (2012). STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell, 22(4), 466–478.
Huang, B., Zhao, J., Shen, S., Li, H., He, K. L., Shen, G. X., et al. (2007). Listeria monocytogenes promotes tumor growth via tumor cell toll-like receptor 2 signaling. Cancer Research, 67(9), 4346–4352.
Szajnik, M., Szczepanski, M. J., Czystowska, M., Elishaev, E., Mandapathil, M., Nowak-Markwitz, E., et al. (2009). TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene, 28, 4353–4563.
Cherfils-Vicini, J., Platonova, S., Gillard, M., Laurans, L., Validire, P., Caliandro, R., et al. (2010). Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. Journal of Clinical Investigation, 120, 1285–1297.
Chiba, S., Baghdadi, M., Akiba, H., Yoshiyama, H., Kinoshita, I., Dosaka-Akita, H., et al. (2012). Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nature Immunology, 13, 832–842.
Tang, D., & Lotze, M. T. (2012). Tumor immunity times out: TIM-3 and HMGB1. Nature Immunology, 9, 808–810.
Green, D. R., et al. (2009). Immunogenic and tolerogenic cell death. Nature Reviews Immunology, 9, 353–363.
Obeid, M., et al. (2007). Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature Medicine, 13, 54–61.
Jinushi, M., et al. (2009). Milk fat globule EGF-8 triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. Journal of Experimental Medicine, 206, 1317–1326.
Loges, S., Schmidt, T., Tjwa, M., van Geyte, K., Lievens, D., Lutgens, E., et al. (2010). Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood, 115(11), 2264–2273.
Elliott, M. R., Chekeni, F. B., Trampont, P. C., Lazarowski, E. R., Kadl, A., Walk, S. F., et al. (2009). Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature, 461(7261), 282–286.
Chao, M. P., Majeti, R., & Weissman, I. L. (2011). Programmed cell removal: a new obstacle in the road to developing cancer. Nature Reviews Cancer, 12, 58–67.
Chao, M. P., Jaiswal, S., Weissman-Tsukamoto, R., Alizadeh, A. A., Gentles, A. J., Volkmer, J., et al. (2010). Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and counterbalanced by CD47. Science Translational Medicine, 2(63), 63ra94.
Jaiswal, S., Jamieson, C. H., Pang, W. W., Park, C. Y., Chao, M. P., Majeti, R., et al. (2009). CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell, 138(2), 271–285.
Baghdadi, M., Yoneda, A., Yamashina, T., Nagao, H., Komohara, Y., Nagai, S., et al. (2013). TIM-4 glycoprotein-mediated degradation of dying tumor cells by autophagy leads to reduced antigen presentation and increased immune tolerance. Immunity, 39, 1070–1081.
Jinushi, M., Chiba, S., Baghdadi, M., Kinoshita, I., Dosaka-Akita, H., Ito, K., et al. (2012). ATM-mediated DNA damage signals mediate immune escape through integrin-αvβ3-dependent mechanisms. Cancer Research, 72(1), 56–65.
Di Micco, R., Fumagalli, M., Cicalese, A., Piccinin, S., Gasparini, P., Luise, C., et al. (2006). Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature, 444, 638–642.
Mooi, W. J., & Peeper, D. S. (2006). Oncogene-induced cell senescence—halting on the road to cancer. New England Journal of Medicine, 355, 1037–1046.
Collado, M., Blasco, M. A., & Serrano, M. (2007). Cellular senescence in cancer and aging. Cell, 130, 223–233.
Kuilman, T., & Peeper, D. S. (2009). Senescence-messaging secretome: SMS-ing cellular stress. Nature Reviews Cancer, 9(2), 81–94.
Coppé, J. P., Desprez, P. Y., Krtolica, A., & Campisi, J. (2010). The senescence-associated secretory phenotype: the dark side of tumor suppression. Annual Review of Pathology, 5, 99–118.
Acosta, J. C., Banito, A., Wuestefeld, T., Georgilis, A., Janich, P., & Morton, J. P. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology, 8, 978–990.
Kuilman, T., Michaloglou, C., Vredeveld, L. C., Douma, S., van Doorn, R., Desmet, C. J., et al. (2008). Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell, 133(6), 1019–1031.
Pazolli, E., Alspach, E., Milczarek, A., Prior, J., Piwnica-Worms, D., & Stewart, S. A. (2012). Chromatin remodeling underlies the senescence-associated secretory phenotype of tumor stromal fibroblasts that supports cancer progression. Cancer Research, 72(9), 225122–225161.
Canino, C., Mori, F., Cambria, A., Diamantini, A., Germoni, S., Alessandrini, G., et al. (2012). SASP mediates chemoresistance and tumor-initiating-activity of mesothelioma cells. Oncogene, 31(26), 3148–3163.
Yoshimoto, S., Loo, T. M., Atarashi, K., Kanda, H., Sato, S., Oyadomari, S., et al. (2013). Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature, 499, 97–101.
Lujambio, A., Akkari, L., Simon, J., Grace, D., Tschaharganeh, D. F., & Bolden, J. E. (2013). Non-cell-autonomous tumor suppression by p53. Cell, 153, 449–460.
Xue, W., Zender, L., Miething, C., Dickins, R. A., Hernando, E., Krizhanovsky, V., et al. (2007). Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature, 445(7128), 656–660.
Chien, Y., Scuoppo, C., Wang, X., Fang, X., Balgley, B., Bolden, J. E., et al. (2012). Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes and Development, 25(20), 2125–2136.
Formenti, S. C., & Demaria, S. (2009). Systemic effects of local radiotherapy. Lancet Oncology, 10(7), 718–726.
Klug, F., Prakash, H., Huber, P. E., Seibel, T., Bender, N., Halama, N., et al. (2013). Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell, 24(5), 589–602.
Burnette, B. C., Liang, H., Lee, Y., Chlewicki, L., Khodarev, N. N., Weichselbaum, R. R., et al. (2011). The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Research, 71(7), 2488–2496.
Lee, Y., Auh, S. L., Wang, Y., Burnette, B., Wang, Y., Meng, Y., et al. (2009). Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood, 114(3), 589–595.
Takeshima, T., Chamoto, K., Wakita, D., Ohkuri, T., Togashi, Y., Shirato, H., et al. (2010). Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Research, 70(7), 2697–2706.
Ludgate, C. M. (2012). Optimizing cancer treatments to induce an acute immune response: radiation Abscopal effects, PAMPs, and DAMPs. Clinical Cancer Research, 18, 4522–4525.
Apetoh, L., Ghiringhelli, F., Tesniere, A., Criollo, A., Ortiz, C., Lidereau, R., et al. (2007). The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunology Reviews, 220, 47–59.
Krysko, D. V., Garg, A. D., Kaczmarek, A., Krysko, O., Agostinis, P., & Vandenabeele, P. (2012). Immunogenic cell death and DAMPs in cancer therapy. Nature Reviews Cancer, 12, 860–875.
Kozin, S. V., Duda, D. G., Munn, L. L., & Jain, R. K. (2012). Neovascularization after irradiation: what is the source of newly formed vessels in recurring tumors? Journal of the National Cancer Institute, 104, 899–905.
Xu, J., Escamilla, J., Mok, S., David, J., Priceman, S., West, B., et al. (2013). CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Research, 73(9), 2782–2794.
Sharma, S. V., Bell, D. W., Settleman, J., & Haber, D. A. (2007). Epidermal growth factor receptor mutations in lung cancer. Nature Reviews Cancer, 7(3), 169–181.
Lynch, T. J., Bell, D. W., Sordella, R., Gurubhagavatula, S., Okimoto, R. A., Brannigan, B. W., et al. (2004). Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. New England Journal of Medicine, 350(21), 2129–2139.
Kobayashi, S., Boggon, T. J., Dayaram, T., Jänne, P. A., Kocher, O., Meyerson, M., et al. (2005). EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. New England Journal of Medicine, 352(8), 786–792.
Bivona, T. G., Hieronymus, H., Parker, J., Chang, K., Taron, M., Rosell, R., et al. (2011). FAS and NF-κB signalling modulate dependence of lung cancers on mutant EGFR. Nature, 47, 523–526.
Engelman, J. A., Zejnullahu, K., Mitsudomi, T., Song, Y., Hyland, C., Park, J. O., et al. (2007). MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science, 316, 1039–1043.
Vanneman, M., & Dranoff, G. (2012). Combining immunotherapy and targeted therapies in cancer treatment. Nature Reviews Cancer, 12(4), 237–251.
Gao, S. P., Mark, K. G., Leslie, K., Pao, W., Motoi, N., Gerald, W. L., et al. (2007). Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. Journal of Clinical Investigation, 117, 3846–3856.
Jinushi, M., Chiba, S., Yoshiyama, H., Masutomi, K., Kinoshita, I., Dosaka-Akita, H., et al. (2011). Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proceedings of the National Academy of Sciences of the United States of America, 108(30), 12425–12430.
Zhang, Z., Lee, J. C., Lin, L., Olivas, V., Au, V., LaFramboise, T., et al. (2012). Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nature Genetics, 44(8), 852–860.
Chapman, P. B., Hauschild, A., Robert, C., Haanen, J. B., Ascierto, P., Larkin, J., BRIM-3 Study Group, et al. (2011). Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New England Journal of Medicine, 364, 2507–2516.
Sumimoto, H., Imabayashi, F., Iwata, T., & Kawakami, Y. (2006). The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. Journal of Experimental Medicine, 203, 1651–1656.
Jiang, X., Zhou, J., Giobbie-Hurder, A., Wargo, J., & Hodi, F. S. (2013). The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clinical Cancer Research, 19, 598–609.
Knight, D. A., Ngiow, S. F., Li, M., Parmenter, T., Mok, S., Cass, A., et al. (2013). Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. Journal of Clinical Investigation, 123, 1371–1381.
Frederick, D. T., Piris, A., Cogdill, A. P., Cooper, Z. A., Lezcano, C., Ferrone, C. R., et al. (2013). BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clinical Cancer Research, 19, 1225–1231.
Hudes, G., Carducci, M., Tomczak, P., Dutcher, J., Figlin, R., Kapoor, A., Global ARCC Trial, et al. (2007). Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. New England Journal of Medicine, 356, 2271–2281.
Pearce, E. L., & Pearce, E. J. (2013). Metabolic pathways in immune cell activation and quiescence. Immunity, 38, 633–643.
Waickman, A. T., & Powell, J. D. (2012). mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunology Reviews, 49, 43–58.
Amiel, E., Everts, B., Freitas, T. C., King, I. L., Curtis, J. D., Pearce, E. L., et al. (2012). Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. Journal of Immunology, 189, 2151–2158.
Berezhnoy, A., Castro, I., Levay, A., Malek, T. R., & Gilboa, E. (2014). Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. Journal of Clinical Investigation, 124, 188–197.
Bianchini, G., & Gianni, L. (2014). The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncology, 2, e58–e68.
Taylor, C., Hershman, D., Shah, N., Suciu-Foca, N., Petrylak, D. P., Taub, R., et al. (2007). Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clinical Cancer Research, 13, 5133–5143.
DeNardo, D. G., Brennan, D. J., Rexhepaj, E., Ruffell, B., Shiao, S. L., Madden, S. F., et al. (2011). Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discovery, 1(1), 54–67.
Kohrt, H. E., Nouri, N., Nowels, K., Johnson, D., Holmes, S., & Lee, P. P. (2005). Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Medicine, 2(9), e284.
Hideshima, T., Mitsiades, C., Tonon, G., Richardson, P. G., & Anderson, K. C. (2007). Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nature Reviews Cancer, 8, 585–598.
Richardson, P. G., Sonneveld, P., Schuster, M. W., Irwin, D., Stadtmauer, E. A., Facon, T., Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators, et al. (2005). Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. New England Journal of Medicine, 352, 2487–2498.
Palumbo, A., Hajek, R., Delforge, M., Kropff, M., Petrucci, M. T., Catalano, J., MM-015 Investigators, et al. (2012). Continuous lenalidomide treatment for newly diagnosed multiple myeloma. New England Journal of Medicine, 366, 1759–1769.
Chauhan, D., Singh, A. V., Brahmandam, M., Carrasco, R., Bandi, M., Hideshima, T., et al. (2009). Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell, 16, 309–323.
Jinushi, M., Vanneman, M., Munshi, N. C., Tai, Y. T., Prabhala, R. H., Ritz, J., et al. (2008). MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proceedings of the National Academy of Sciences of the United States of America, 105, 1285–1290.
Martiniani, R., Di Loreto, V., Di Sano, C., Lombardo, A., & Liberati, A. M. (2012). Biological activity of lenalidomide and its underlying therapeutic effects in multiple myeloma. Advances in Hematology, 2012, 842945.
Ghiringhelli, F., Apetoh, L., Tesniere, A., Aymeric, L., Ma, Y., Ortiz, C., et al. (2009). Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nature Medicine, 15, 1170–1178.
Yamamoto, R., Nishikori, M., Tashima, M., Sakai, T., Ichinohe, T., Takaori-Kondo, A., et al. (2009). B7-H1 expression is regulated by MEK/ERK signaling pathway in anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Science, 100, 2093–2100.
Ribas, A., & Wolchok, J. D. (2013). Combining cancer immunotherapy and targeted therapy. Current Opinion in Immunology, 25, 291–296.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jinushi, M. Immune regulation of therapy-resistant niches: emerging targets for improving anticancer drug responses. Cancer Metastasis Rev 33, 737–745 (2014). https://doi.org/10.1007/s10555-014-9501-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-014-9501-9