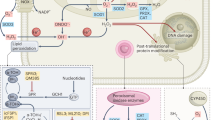
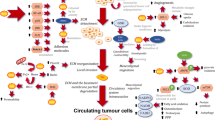
Abstract
According to a “canonical” view, reactive oxygen species (ROS) positively contribute, in different ways, to carcinogenesis and to malignant progression of tumor cells: they drive genomic damage and genetic instability, transduce, as signaling intermediates, mitogenic and survival inputs by growth factor receptors and adhesion molecules, promote cell motility and shape the tumor microenvironment by inducing inflammation/repair and angiogenesis. Chemopreventive and tumor-inhibitory effects of endogenous, diet-derived or supplemented antioxidants largely support this notion. However, emerging lines of evidence indicates that tumor cells also need to defend themselves from oxidative damage in order to survive and successfully spread at distance. This “heresy” has recently received important impulse from studies on the role of antioxidant capacity in cancer stem cells self-renewal and resistance to therapy; additionally, the transforming activity of some oncogenes has been unexpectedly linked to their capacity to maintain elevated intracellular levels of reduced glutathione (GSH), the principal redox buffer. These studies underline the importance of cellular antioxidant capacity in metastasis, as the result of a complex cell program involving enhanced motility and a profound change in energy metabolism. The glycolytic switch (Warburg effect) observed in malignant tissues is triggered by mitochondrial oxidative damage and/or activation of redox-sensitive transcription factors, and results in an increase of cell resistance to oxidants. On the other hand, cytoskeleton rearrangement underlying cell motile and tumor-aggressive behavior use ROS as intermediates and are therefore facilitated by oxidative stress. Along this line of speculation, we suggest that metastasis represents an integrated strategy for cancer cells to avoid oxidative damage and escape excess ROS in the primary tumor site, explaning why redox signaling pathways are often up-regulated in malignancy and metastasis.









Similar content being viewed by others
References
Steeg, P. S. (2006). Tumor metastasis: mechanistic insights and clinical challenges. Nature Medicine, 12, 895–904.
Nguyen, D. X., & Massague, J. (2007). Genetic determinants of cancer metastasis. Nature Reviews Genetics, 8, 341–352.
Chiang, A. C., & Massague, J. (2008). Molecular basis of metastasis. New England Journal of Medicine, 359, 2814–2823.
Fidler, I. J. (2003). The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature Reviews Cancer, 3, 453–458.
Klein, C. A. (2009). Parallel progression of primary tumours and metastases. Nature Reviews Cancer, 9, 302–312.
Mani, S. A., Guo, W., Liao, M. J., Eaton, E. N., Ayyanan, A., Zhou, A. Y., et al. (2008). The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell, 133, 704–715.
Barnhart, B. C., & Simon, M. C. (2007). Metastasis and stem cell pathways. Cancer and Metastasis Reviews, 26, 261–271.
Bertout, J. A., Patel, S. A., & Simon, M. C. (2008). The impact of O2 availability on human cancer. Nature Reviews Cancer, 8, 967–975.
Mantovani, A. (2009). Cancer: inflaming metastasis. Nature, 457, 36–37.
Comoglio, P. M., & Trusolino, L. (2002). Invasive growth: from development to metastasis. Journal of Clinical Investigation, 109, 857–862.
Kalluri, R., & Weinberg, R. A. (2009). The basics of epithelial–mesenchymal transition. Journal of Clinical Investigation, 119, 1420–1428.
Etienne-Manneville, S., & Hall, A. (2002). Rho GTPases in cell biology. Nature, 420, 629–635.
Hall, A. (2005). Rho GTPases and the control of cell behaviour. Biochemical Society Transactions, 33, 891–895.
Brakebusch, C., Bouvard, D., Stanchi, F., Sakai, T., & Fassler, R. (2002). Integrins in invasive growth. Journal of Clinical Investigation, 109, 999–1006.
Mitra, S. K., & Schlaepfer, D. D. (2006). Integrin-regulated FAK-Src signaling in normal and cancer cells. Current Opinion in Cell Biology, 18, 516–523.
Raftopoulou, M., Etienne-Manneville, S., Self, A., Nicholls, S., & Hall, A. (2004). Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science, 303, 1179–1181.
Yu, D. H., Qu, C. K., Henegariu, O., Lu, X., & Feng, G. S. (1998). Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. Journal of Biological Chemistry, 273, 21125–21131.
Friedl, P. (2004). Prespecification and plasticity: shifting mechanisms of cell migration. Current Opinion in Cell Biology, 16, 14–23.
Sanz-Moreno, V., Gadea, G., Ahn, J., Paterson, H., Marra, P., Pinner, S., et al. (2008). Rac activation and inactivation control plasticity of tumor cell movement. Cell, 135, 510–523.
Habets, G. G., Scholtes, E. H., Zuydgeest, D., van der Kammen, R. A., Stam, J. C., Berns, A., et al. (1994). Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell, 77, 537–549.
Clark, E. A., Golub, T. R., Lander, E. S., & Hynes, R. O. (2000). Genomic analysis of metastasis reveals an essential role for RhoC. Nature, 406, 532–535.
Murakami, M., Meneses, P. I., Knight, J. S., Lan, K., Kaul, R., Verma, S. C., et al. (2008). Nm23-H1 modulates the activity of the guanine exchange factor Dbl-1. International Journal of Cancer, 123, 500–510.
Birchmeier, C., Birchmeier, W., Gherardi, E., & Vande Woude, G. F. (2003). Met, metastasis, motility and more. Nature Reviews Molecular Cell Biology, 4, 915–925.
Douma, S., Van, L. T., Zevenhoven, J., Meuwissen, R., Van, G. E., & Peeper, D. S. (2004). Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature, 430, 1034–1039.
Geiger, T. R., & Peeper, D. S. (2009). Metastasis mechanisms. Biochimica et Biophysica Acta, 1796, 293–308.
Wei, L., Yang, Y., Zhang, X., & Yu, Q. (2004). Altered regulation of Src upon cell detachment protects human lung adenocarcinoma cells from anoikis. Oncogene, 23, 9052–9061.
Aguirre-Ghiso, J. A. (2007). Models, mechanisms and clinical evidence for cancer dormancy. Nature Reviews Cancer, 7, 834–846.
Kim, S., Takahashi, H., Lin, W. W., Descargues, P., Grivennikov, S., Kim, Y., et al. (2009). Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature, 457, 102–106.
Kaplan, R. N., Riba, R. D., Zacharoulis, S., Bramley, A. H., Vincent, L., Costa, C., et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature, 438, 820–827.
Bates, R. C., & Mercurio, A. M. (2003). Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Molecular Biology of the Cell, 14, 1790–1800.
Luo, J. L., Tan, W., Ricono, J. M., Korchynskyi, O., Zhang, M., Gonias, S. L., et al. (2007). Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature, 446, 690–694.
Kaelin, W. G., Jr., & Ratcliffe, P. J. (2008). Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Molecular Cell, 30, 393–402.
Pouyssegur, J., Dayan, F., & Mazure, N. M. (2006). Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature, 441, 437–443.
Kim, J. W., Tchernyshyov, I., Semenza, G. L., & Dang, C. V. (2006). HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab, 3, 177–185.
Fukuda, R., Zhang, H., Kim, J. W., Shimoda, L., Dang, C. V., & Semenza, G. L. (2007). HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell, 129, 111–122.
Dewhirst, M. W., Cao, Y., & Moeller, B. (2008). Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nature Reviews Cancer, 8, 425–437.
Pennacchietti, S., Michieli, P., Galluzzo, M., Mazzone, M., Giordano, S., & Comoglio, P. M. (2003). Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell, 3, 347–361.
Erler, J. T., Bennewith, K. L., Nicolau, M., Dornhofer, N., Kong, C., Le, Q. T., et al. (2006). Lysyl oxidase is essential for hypoxia-induced metastasis. Nature, 440, 1222–1226.
Denko, N. C. (2008). Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nature Reviews Cancer., 8, 705–13.
Vander Heiden, M. G., Cantley, L. C., & Thompson, C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science, 324, 1029–1033.
Hsu, P. P., & Sabatini, D. M. (2008). Cancer cell metabolism: Warburg and beyond. Cell, 134, 703–707.
Gatenby, R. A., & Gawlinski, E. T. (2001). Mathematical models of tumour invasion mediated by transformation-induced alteration of microenvironmental pH. Novartis Foundation Symposium, 240, 85–96.
Rofstad, E. K., Mathiesen, B., Kindem, K., & Galappathi, K. (2006). Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Research, 66, 6699–6707.
Clarke, M. F., Dick, J. E., Dirks, P. B., Eaves, C. J., Jamieson, C. H., Jones, D. L., et al. (2006). Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Research, 66, 9339–9344.
Cipolleschi, M. G., Rovida, E., Ivanovic, Z., Praloran, V., Olivotto, M., & Dello, S. P. (2000). The expansion of murine bone marrow cells preincubated in hypoxia as an in vitro indicator of their marrow-repopulating ability. Leukemia, 14, 735–739.
Simon, M. C., & Keith, B. (2008). The role of oxygen availability in embryonic development and stem cell function. Nature Reviews Molecular Cell Biology, 9, 285–296.
Ezashi, T., Das, P., & Roberts, R. M. (2005). Low O2 tensions and the prevention of differentiation of hES cells. Proceedings of the National Academy of Sciences of the United States of America, 102, 4783–4788.
Kondoh, H., Lleonart, M. E., Gil, J., Wang, J., Degan, P., Peters, G., et al. (2005). Glycolytic enzymes can modulate cellular life span. Cancer Research, 65, 177–185.
Giuntoli, S., Rovida, E., Gozzini, A., Barbetti, V., Cipolleschi, M. G., Olivotto, M., et al. (2007). Severe hypoxia defines heterogeneity and selects highly immature progenitors within clonal erythroleukemia cells. Stem Cells, 25, 1119–1125.
Das, B., Tsuchida, R., Malkin, D., Koren, G., Baruchel, S., & Yeger, H. (2008). Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells, 26, 1818–1830.
Bjerkvig, R., Johansson, M., Miletic, H., & Niclou, S. P. (2009). Cancer stem cells and angiogenesis. Seminars in Cancer Biology, 19, 279–284.
Janssen-Heininger, Y. M., Mossman, B. T., Heintz, N. H., Forman, H. J., Kalyanaraman, B., Finkel, T., et al. (2008). Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radical Biology and Medicine, 45, 1–17.
Murphy, M. P. (2009). How mitochondria produce reactive oxygen species. Biochemical Journal, 417, 1–13.
Paget, M. S., & Buttner, M. J. (2003). Thiol-based regulatory switches. Annual Review of Genetics, 37, 91–121.
Lambeth, J. D. (2004). NOX enzymes and the biology of reactive oxygen. Nature Reviews Cancer, 4, 181–189.
Sundaresan, M., Yu, Z. X., Ferrans, V. J., Irani, K., & Finkel, T. (1995). Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science, 270, 296–299.
Baumer, A. T., Ten, F. H., Sauer, H., Wartenberg, M., Kappert, K., Schnabel, P., et al. (2008). Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for alpha-platelet-derived growth factor receptor-induced production of reactive oxygen species. Journal of Biological Chemistry, 283, 7864–7876.
Lo, Y. Y., & Cruz, T. F. (1995). Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. Journal of Biological Chemistry, 270, 11727–11730.
Ushio-Fukai, M., Zafari, A. M., Fukui, T., Ishizaka, N., & Griendling, K. K. (1996). p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. Journal of Biological Chemistry, 271, 23317–23321.
Chiarugi, P., Pani, G., Giannoni, E., Taddei, L., Colavitti, R., Raugei, G., et al. (2003). Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. Journal of Biological Chemistry, 161, 933–944.
Inoue, M., Sato, E. F., Nishikawa, M., Park, A. M., Kira, Y., Imada, I., et al. (2003). Mitochondrial generation of reactive oxygen species and its role in aerobic life. Current Medicinal Chemistry, 10, 2495–2505.
Nemoto, S., Takeda, K., Yu, Z. X., Ferrans, V. J., & Finkel, T. (2000). Role for mitochondrial oxidants as regulators of cellular metabolism. Molecular and Cellular Biology, 20, 7311–7318.
Guzy, R. D., Hoyos, B., Robin, E., Chen, H., Liu, L., Mansfield, K. D., et al. (2005). Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab, 1, 401–408.
Cai, J., & Jones, D. P. (1998). Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. Journal of Biological Chemistry, 273, 11401–11404.
Giorgio, M., Migliaccio, E., Orsini, F., Paolucci, D., Moroni, M., Contursi, C., et al. (2005). Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell, 122, 221–233.
Pinton, P., Rimessi, A., Marchi, S., Orsini, F., Migliaccio, E., Giorgio, M., et al. (2007). Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science, 315, 659–663.
Pani, G., Koch, O. R., & Galeotti, T. (2009). The p53-p66shc-Manganese Superoxide Dismutase (MnSOD) network: a mitochondrial intrigue to generate reactive oxygen species. International Journal of Biochemistry and Cell Biology, 41, 1002–1005.
Rhee, S. G., Yang, K. S., Kang, S. W., Woo, H. A., & Chang, T. S. (2005). Controlled elimination of intracellular H(2)O(2): regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxidants Redox Signaling, 7, 619–626.
Veal, E. A., Day, A. M., & Morgan, B. A. (2007). Hydrogen peroxide sensing and signaling. Molecular Cell, 26, 1–14.
Chiarugi, P., & Cirri, P. (2003). Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends in Biochemical Sciences, 28, 509–514.
Pani, G., Colavitti, R., Bedogni, B., Anzevino, R., Borrello, S., & Galeotti, T. (2000). A redox signaling mechanism for density-dependent inhibition of cell growth. Journal of Biological Chemistry, 275, 38891–38899.
Mannick, J. B., Hausladen, A., Liu, L., Hess, D. T., Zeng, M., Miao, Q. X., et al. (1999). Fas-induced caspase denitrosylation. Science, 284, 651–654.
Gu, Z., Kaul, M., Yan, B., Kridel, S. J., Cui, J., Strongin, A., et al. (2002). S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science, 297, 1186–1190.
Saitoh, M., Nishitoh, H., Fujii, M., Takeda, K., Tobiume, K., Sawada, Y., et al. (1998). Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO Journal, 17, 2596–2606.
Dinkova-Kostova, A. T., Holtzclaw, W. D., Cole, R. N., Itoh, K., Wakabayashi, N., Katoh, Y., et al. (2002). Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proceedings of the National Academy of Sciences of the United States of America, 99, 11908–11913.
Liu, H., Colavitti, R., Rovira, I. I., & Finkel, T. (2005). Redox-dependent transcriptional regulation. Circulation Research, 97, 967–974.
Xanthoudakis, S., & Curran, T. (1992). Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO Journal, 11, 653–665.
Dansen, T. B., Smits, L. M., van Triest, M. H., de Keizer, P. L., van Leenen, D., Koerkamp, M. G., et al. (2009). Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat Chem Biol, 5, 664–672.
van der Horst, A., Tertoolen, L. G., de Vries-Smits, L. M., Frye, R. A., Medema, R. H., & Burgering, B. M. (2004). FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1). Journal of Biological Chemistry, 279, 28873–28879.
Brunet, A., Bonni, A., Zigmond, M. J., Lin, M. Z., Juo, P., Hu, L. S., et al. (1999). Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell, 96, 857–868.
Nemoto, S., & Finkel, T. (2002). Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science, 295, 2450–2452.
Yasinska, I. M., & Sumbayev, V. V. (2003). S-nitrosation of Cys-800 of HIF-1alpha protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Letters, 549, 105–109.
Gu, J., Milligan, J., & Huang, L. E. (2001). Molecular mechanism of hypoxia-inducible factor 1alpha -p300 interaction. A leucine-rich interface regulated by a single cysteine. Journal of Biological Chemistry, 276, 3550–3554.
Gerald, D., Berra, E., Frapart, Y. M., Chan, D. A., Giaccia, A. J., Mansuy, D., et al. (2004). JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell, 118, 781–794.
Schroedl, C., McClintock, D. S., Budinger, G. R., & Chandel, N. S. (2002). Hypoxic but not anoxic stabilization of HIF-1alpha requires mitochondrial reactive oxygen species. American Journal of Physiology Lung Cellular and Molecular Physiology, 283, L922–L931.
Lu, H., Dalgard, C. L., Mohyeldin, A., Mcfate, T., Tait, A. S., & Verma, A. (2005). Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. Journal of Biological Chemistry, 280, 41928–41939.
Arbiser, J. L., Petros, J., Klafter, R., Govindajaran, B., McLaughlin, E. R., Brown, L. F., et al. (2002). Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proceedings of the National Academy of Sciences of the United States of America, 99, 715–720.
Wang, F. S., Wang, C. J., Chen, Y. J., Chang, P. R., Huang, Y. T., Sun, Y. C., et al. (2004). Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1alpha and VEGF-A expression in shock wave-stimulated osteoblasts. Journal of Biological Chemistry, 279, 10331–10337.
Gao, P., Zhang, H., Dinavahi, R., Li, F., Xiang, Y., Raman, V., et al. (2007). HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell, 12, 230–238.
Nott, A., Watson, P. M., Robinson, J. D., Crepaldi, L., & Riccio, A. (2008). S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature, 455, 411–415.
Bedogni, B., Pani, G., Colavitti, R., Riccio, A., Borrello, S., Murphy, M., et al. (2003). Redox regulation of cAMP-responsive element-binding protein and induction of manganous superoxide dismutase in nerve growth factor-dependent cell survival. Journal of Biological Chemistry, 278, 16510–16519.
Riccio, A., Alvania, R. S., Lonze, B. E., Ramanan, N., Kim, T., Huang, Y., et al. (2006). A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Molecular Cell, 21, 283–294.
Ito, K., Hanazawa, T., Tomita, K., Barnes, P. J., & Adcock, I. M. (2004). Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochemical and Biophysical Research Communications, 315, 240–245.
Moldovan, L., Irani, K., Moldovan, N. I., Finkel, T., & Goldschmidt-Clermont, P. J. (1999). The actin cytoskeleton reorganization induced by Rac1 requires the production of superoxide. Antioxidants Redox Signaling, 1, 29–43.
Joneson, T., & Bar-Sagi, D. (1998). A Rac1 effector site controlling mitogenesis through superoxide production. Journal of Biological Chemistry, 273, 17991–17994.
Kheradmand, F., Werner, E., Tremble, P., Symons, M., & Werb, Z. (1998). Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science, 280, 898–902.
Werner, E., & Werb, Z. (2002). Integrins engage mitochondrial function for signal transduction by a mechanism dependent on Rho GTPases. Journal of Cell Biology, 158, 357–368.
Zhou, C., Ziegler, C., Birder, L. A., Stewart, A. F., & Levitan, E. S. (2006). Angiotensin II and stretch activate NADPH oxidase to destabilize cardiac Kv4.3 channel mRNA. Circulation Research, 98, 1040–1047.
Taddei, M. L., Parri, M., Mello, T., Catalano, A., Levine, A. D., Raugei, G., et al. (2007). Integrin-mediated cell adhesion and spreading engage different sources of reactive oxygen species. Antioxidants Redox Signaling, 9, 469–481.
Nimnual, A. S., Taylor, L. J., & Bar-Sagi, D. (2003). Redox-dependent downregulation of Rho by Rac. Nature Cell Biology, 5, 236–241.
Ng, J., & Luo, L. (2004). Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron, 44, 779–793.
Zondag, G. C., Evers, E. E., ten Klooster, J. P., Janssen, L., van der Kammen, R. A., & Collard, J. G. (2000). Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial–mesenchymal transition. Journal of Cell Biology, 149, 775–782.
Buricchi, F., Giannoni, E., Grimaldi, G., Parri, M., Raugei, G., Ramponi, G., et al. (2007). Redox regulation of ephrin/integrin cross-talk. Cell Adh Migr, 1, 33–42.
Taddei, M. L., Parri, M., Angelucci, A., Onnis, B., Bianchini, F., Giannoni, E., et al. (2009). Kinase-dependent and -independent roles of EphA2 in the regulation of prostate cancer invasion and metastasis. American Journal of Pathology, 174, 1492–1503.
Giannoni, E., Buricchi, F., Raugei, G., Ramponi, G., & Chiarugi, P. (2005). Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Molecular and Cellular Biology, 25, 6391–6403.
Fratelli, M., Demol, H., Puype, M., Casagrande, S., Eberini, I., Salmona, M., et al. (2002). Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proceedings of the National Academy of Sciences of the United States of America, 99, 3505–3510.
Fiaschi, T., Cozzi, G., Raugei, G., Formigli, L., Ramponi, G., & Chiarugi, P. (2006). Redox regulation of beta-actin during integrin-mediated cell adhesion. Journal of Biological Chemistry, 281, 22983–22991.
Terman, J. R., Mao, T., Pasterkamp, R. J., Yu, H. H., & Kolodkin, A. L. (2002). MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell, 109, 887–900.
Ventura, A., & Pelicci, P. G. (2002). Semaphorins: green light for redox signaling? Sci STKE, 2002, e44.
Suzuki, T., Nakamoto, T., Ogawa, S., Seo, S., Matsumura, T., Tachibana, K., et al. (2002). MICAL, a novel CasL interacting molecule, associates with vimentin. Journal of Biological Chemistry, 277, 14933–14941.
Nadella, M., Bianchet, M. A., Gabelli, S. B., Barrila, J., & Amzel, L. M. (2005). Structure and activity of the axon guidance protein MICAL. Proceedings of the National Academy of Sciences of the United States of America, 102, 16830–16835.
Fischer, J., Weide, T., & Barnekow, A. (2005). The MICAL proteins and rab1: a possible link to the cytoskeleton? Biochemical and Biophysical Research Communications, 328, 415–423.
Kanda, I., Nishimura, N., Nakatsuji, H., Yamamura, R., Nakanishi, H., & Sasaki, T. (2008). Involvement of Rab13 and JRAB/MICAL-L2 in epithelial cell scattering. Oncogene, 27, 1687–1695.
Ferraro, D., Corso, S., Fasano, E., Panieri, E., Santangelo, R., Borrello, S., et al. (2006). Pro-metastatic signaling by c-Met through RAC-1 and reactive oxygen species (ROS). Oncogene, 25, 3689–3698.
Jagadeeswaran, R., Jagadeeswaran, S., Bindokas, V. P., & Salgia, R. (2007). Activation of HGF/c-Met pathway contributes to the reactive oxygen species generation and motility of small cell lung cancer cells. American Journal of Physiology Lung Cellular and Molecular Physiology, 292, L1488–L1494.
Li, W., Liu, G., Chou, I. N., & Kagan, H. M. (2000). Hydrogen peroxide-mediated, lysyl oxidase-dependent chemotaxis of vascular smooth muscle cells. Journal of Cellular Biochemistry, 78, 550–557.
Ushio-Fukai, M. (2009). Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxidants Redox Signaling, 11, 1289–1299.
Wu, R. F., Xu, Y. C., Ma, Z., Nwariaku, F. E., Sarosi, G. A., Jr., & Terada, L. S. (2005). Subcellular targeting of oxidants during endothelial cell migration. Journal of Cell Biology, 171, 893–904.
Diaz, B., Shani, G., Pass, I., Anderson, D., Quintavalle, M., & Courtneidge, S. A. (2009). Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal, 2, ra53.
Gianni, D., Diaz, B., Taulet, N., Fowler, B., Courtneidge, S. A., & Bokoch, G. M. (2009). Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Science Signal, 2, ra54.
Patel, H. H., & Insel, P. A. (2009). Lipid rafts and caveolae and their role in compartmentation of redox signaling. Antioxidants Redox Signaling, 11, 1357–1372.
Gaus, K., Le, L. S., Balasubramanian, N., & Schwartz, M. A. (2006). Integrin-mediated adhesion regulates membrane order. Journal of Cell Biology, 174, 725–734.
Meng, T. C., Fukada, T., & Tonks, N. K. (2002). Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Molecular Cell, 9, 387–399.
Campbell, M., Anderson, P., & Trimble, E. R. (2008). Glucose lowers the threshold for human aortic vascular smooth muscle cell migration: inhibition by protein phosphatase-2A. Diabetologia, 51, 1068–1080.
Leopold, J. A., Walker, J., Scribner, A. W., Voetsch, B., Zhang, Y. Y., Loscalzo, A. J., et al. (2003). Glucose-6-phosphate dehydrogenase modulates vascular endothelial growth factor-mediated angiogenesis. Journal of Biological Chemistry, 278, 32100–32106.
Ames, B. N., Gold, L. S., & Willett, W. C. (1995). The causes and prevention of cancer. Proceedings of the National Academy of Sciences of the United States of America, 92, 5258–5265.
Ambrosone, C. B., Freudenheim, J. L., Thompson, P. A., Bowman, E., Vena, J. E., Marshall, J. R., et al. (1999). Manganese superoxide dismutase (MnSOD) genetic polymorphisms, dietary antioxidants, and risk of breast cancer. Cancer Research, 59, 602–606.
Ratnasinghe, D., Tangrea, J. A., Andersen, M. R., Barrett, M. J., Virtamo, J., Taylor, P. R., et al. (2000). Glutathione peroxidase codon 198 polymorphism variant increases lung cancer risk. Cancer Research, 60, 6381–6383.
Zhao, Y., Oberley, T. D., Chaiswing, L., Lin, S. M., Epstein, C. J., Huang, T. T., et al. (2002). Manganese superoxide dismutase deficiency enhances cell turnover via tumor promoter-induced alterations in AP-1 and p53-mediated pathways in a skin cancer model. Oncogene, 21, 3836–3846.
Marnett, L. J. (2000). Oxyradicals and DNA damage. Carcinogenesis, 21, 361–370.
Hussain, S. P., Hofseth, L. J., & Harris, C. C. (2003). Radical causes of cancer. Nature Reviews Cancer, 3, 276–285.
Cerutti, P. A. (1985). Prooxidant states and tumor promotion. Science, 227, 375–381.
Irani, K., Xia, Y., Zweier, J. L., Sollott, S. J., Der, C. J., Fearon, E. R., et al. (1997). Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science, 275, 1649–1652.
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100, 57–70.
Szatrowski, T. P., & Nathan, C. F. (1991). Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Research, 51, 794–798.
Lim, S. D., Sun, C., Lambeth, J. D., Marshall, F., Amin, M., Chung, L., et al. (2005). Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate, 62, 200–207.
Mitsushita, J., Lambeth, J. D., & Kamata, T. (2004). The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Research, 64, 3580–3585.
Shinohara, M., Shang, W. H., Kubodera, M., Harada, S., Mitsushita, J., Kato, M., et al. (2007). Nox1 redox signaling mediates oncogenic Ras-induced disruption of stress fibers and focal adhesions by down-regulating Rho. Journal of Biological Chemistry, 282, 17640–17648.
Lander, H. M., Milbank, A. J., Tauras, J. M., Hajjar, D. P., Hempstead, B. L., Schwartz, G. D., et al. (1996). Redox regulation of cell signalling. Nature, 381, 380–381.
Benhar, M., Dalyot, I., Engelberg, D., & Levitzki, A. (2001). Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Molecular and Cellular Biology, 21, 6913–6926.
Rhee, S. G., Bae, Y. S., Lee, S. R., & Kwon, J. (2000). Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE, 2000, e1.
Konishi, H., Tanaka, M., Takemura, Y., Matsuzaki, H., Ono, Y., Kikkawa, U., et al. (1997). Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proceedings of the National Academy of Sciences of the United States of America, 94, 11233–11237.
Pani, G., Bedogni, B., Colavitti, R., Anzevino, R., Borrello, S., & Galeotti, T. (2001). Cell compartmentalization in redox signaling. IUBMB Life, 52, 7–16.
Coats, S., Flanagan, W. M., Nourse, J., & Roberts, J. M. (1996). Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science, 272, 877–880.
Medema, R. H., Kops, G. J., Bos, J. L., & Burgering, B. M. (2000). AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature, 404, 782–787.
Nogueira, V., Park, Y., Chen, C. C., Xu, P. Z., Chen, M. L., Tonic, I., et al. (2008). Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell, 14, 458–470.
Itahana, K., Campisi, J., & Dimri, G. P. (2004). Mechanisms of cellular senescence in human and mouse cells. Biogerontology, 5, 1–10.
Parrinello, S., Samper, E., Krtolica, A., Goldstein, J., Melov, S., & Campisi, J. (2003). Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nature Cell Biology, 5, 741–747.
Packer, L., & Fuehr, K. (1977). Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature, 267, 423–425.
Bell, E. L., Klimova, T. A., Eisenbart, J., Schumacker, P. T., & Chandel, N. S. (2007). Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Molecular and Cellular Biology, 27, 5737–5745.
Bermudez, Y., Ahmadi, S., Lowell, N. E., & Kruk, P. A. (2007). Vitamin E suppresses telomerase activity in ovarian cancer cells. Cancer Detection and Prevention, 31, 119–128.
Kwon, J., Lee, S. R., Yang, K. S., Ahn, Y., Kim, Y. J., Stadtman, E. R., et al. (2004). Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proceedings of the National Academy of Sciences of the United States of America, 101, 16419–16424.
Romashkova, J. A., & Makarov, S. S. (1999). NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature, 401, 86–90.
Ozes, O. N., Mayo, L. D., Gustin, J. A., Pfeffer, S. R., Pfeffer, L. M., & Donner, D. B. (1999). NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature, 401, 82–85.
Anderson, M. T., Staal, F. J., Gitler, C., Herzenberg, L. A., & Herzenberg, L. A. (1994). Separation of oxidant-initiated and redox-regulated steps in the NF-kappa B signal transduction pathway. Proceedings of the National Academy of Sciences of the United States of America, 91, 11527–11531.
Sulciner, D. J., Irani, K., Yu, Z. X., Ferrans, V. J., Goldschmidt-Clermont, P., & Finkel, T. (1996). rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-kappaB activation. Molecular and Cellular Biology, 16, 7115–7121.
Yang, J. Q., Zhao, W., Duan, H., Robbins, M. E., Buettner, G. R., Oberley, L. W., et al. (2001). v-Ha-RaS oncogene upregulates the 92-kDa type IV collagenase (MMP-9) gene by increasing cellular superoxide production and activating NF-kappaB. Free Radical Biology and Medicine, 31, 520–529.
Joneson, T., & Bar-Sagi, D. (1999). Suppression of Ras-induced apoptosis by the Rac GTPase. Molecular and Cellular Biology, 19, 5892–5901.
Lluis, J. M., Buricchi, F., Chiarugi, P., Morales, A., & Fernandez-Checa, J. C. (2007). Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer Research, 67, 7368–7377.
Barbie, D. A., Tamayo, P., Boehm, J. S., Kim, S. Y., Moody, S. E., Dunn, I. F., et al. (2009). Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature, 462, 108–112.
Mayo, M. W., Wang, C. Y., Cogswell, P. C., Rogers-Graham, K. S., Lowe, S. W., Der, C. J., et al. (1997). Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science, 278, 1812–1815.
Ciani, E., Guidi, S., Della, V. G., Perini, G., Bartesaghi, R., & Contestabile, A. (2002). Nitric oxide protects neuroblastoma cells from apoptosis induced by serum deprivation through cAMP-response element-binding protein (CREB) activation. Journal of Biological Chemistry, 277, 49896–49902.
Hess, D. T., Matsumoto, A., Kim, S. O., Marshall, H. E., & Stamler, J. S. (2005). Protein S-nitrosylation: purview and parameters. Nature Reviews Molecular Cell Biology, 6, 150–166.
Pan, S., & Berk, B. C. (2007). Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circulation Research, 100, 213–219.
Giannoni, E., Fiaschi, T., Ramponi, G., & Chiarugi, P. (2009). Redox regulation of anoikis resistance of metastatic prostate cancer cells: key role for Src and EGFR-mediated pro-survival signals. Oncogene, 28, 2074–2086.
Pani, G., Giannoni, E., Galeotti, T., & Chiarugi, P. (2009). Redox-based escape mechanism from death: the cancer lesson. Antioxidants Redox Signaling, 11, 2791–2806.
Maulik, N. (2002). Redox signaling of angiogenesis. Antioxidants Redox Signaling, 4, 805–815.
Komatsu, D., Kato, M., Nakayama, J., Miyagawa, S., & Kamata, T. (2008). NADPH oxidase 1 plays a critical mediating role in oncogenic Ras-induced vascular endothelial growth factor expression. Oncogene, 27, 4724–4732.
Zundel, W., Schindler, C., Haas-Kogan, D., Koong, A., Kaper, F., Chen, E., et al. (2000). Loss of PTEN facilitates HIF-1-mediated gene expression. Genes and Development, 14, 391–396.
Hudson, C. C., Liu, M., Chiang, G. G., Otterness, D. M., Loomis, D. C., Kaper, F., et al. (2002). Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Molecular and Cellular Biology, 22, 7004–7014.
Sarbassov, D. D., & Sabatini, D. M. (2005). Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. Journal of Biological Chemistry, 280, 39505–39509.
Metzen, E., Zhou, J., Jelkmann, W., Fandrey, J., & Brune, B. (2003). Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Molecular Biology of the Cell, 14, 3470–3481.
Kasuno, K., Takabuchi, S., Fukuda, K., Kizaka-Kondoh, S., Yodoi, J., Adachi, T., et al. (2004). Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. Journal of Biological Chemistry, 279, 2550–2558.
Parenti, A., Morbidelli, L., Cui, X. L., Douglas, J. G., Hood, J. D., Granger, H. J., et al. (1998). Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. Journal of Biological Chemistry, 273, 4220–4226.
Colavitti, R., Pani, G., Bedogni, B., Anzevino, R., Borrello, S., Waltenberger, J., et al. (2002). Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. Journal of Biological Chemistry, 277, 3101–3108.
Harfouche, R., Malak, N. A., Brandes, R. P., Karsan, A., Irani, K., & Hussain, S. N. (2005). Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. FASEB Journal, 19, 1728–1730.
Ushio-Fukai, M. (2006). Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovascular Research, 71, 226–235.
Urao, N., Inomata, H., Razvi, M., Kim, H. W., Wary, K., McKinney, R., et al. (2008). Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circulation Research, 103, 212–220.
Lelkes, P. I., Hahn, K. L., Sukovich, D. A., Karmiol, S., & Schmidt, D. H. (1998). On the possible role of reactive oxygen species in angiogenesis. Advances in Experimental Medicine and Biology, 454, 295–310.
Fukumura, D., Kashiwagi, S., & Jain, R. K. (2006). The role of nitric oxide in tumour progression. Nature Reviews Cancer, 6, 521–534.
Radisky, D. C., Levy, D. D., Littlepage, L. E., Liu, H., Nelson, C. M., Fata, J. E., et al. (2005). Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature, 436, 123–127.
Nelson, C. M., Khauv, D., Bissell, M. J., & Radisky, D. C. (2008). Change in cell shape is required for matrix metalloproteinase-induced epithelial-mesenchymal transition of mammary epithelial cells. Journal of Cellular Biochemistry, 105, 25–33.
Cannito, S., Novo, E., Compagnone, A., di Valfre, B. L., Busletta, C., Zamara, E., et al. (2008). Redox mechanisms switch on hypoxia-dependent epithelial–mesenchymal transition in cancer cells. Carcinogenesis, 29, 2267–2278.
Ishikawa, K., Takenaga, K., Akimoto, M., Koshikawa, N., Yamaguchi, A., Imanishi, H., et al. (2008). ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science, 320, 661–664.
Binker, M. G., Binker-Cosen, A. A., Richards, D., Oliver, B., & Cosen-Binker, L. I. (2009). EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochemical and Biophysical Research Communications, 379, 445–450.
Oberley, T. D., Schultz, J. L., Li, N., & Oberley, L. W. (1995). Antioxidant enzyme levels as a function of growth state in cell culture. Free Radical Biology and Medicine, 19, 53–65.
Trachootham, D., Alexandre, J., & Huang, P. (2009). Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov, 8, 579–591.
Lee, A. C., Fenster, B. E., Ito, H., Takeda, K., Bae, N. S., Hirai, T., et al. (1999). Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. Journal of Biological Chemistry, 274, 7936–7940.
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D., & Lowe, S. W. (1997). Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell, 88, 593–602.
Takahashi, A., Ohtani, N., Yamakoshi, K., Iida, S., Tahara, H., Nakayama, K., et al. (2006). Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nature Cell Biology, 8, 1291–1297.
Lowe, S. W., Jacks, T., Housman, D. E., & Ruley, H. E. (1994). Abrogation of oncogene-associated apoptosis allows transformation of p53-deficient cells. Proceedings of the National Academy of Sciences of the United States of America, 91, 2026–2030.
Pani, G., Bedogni, B., Anzevino, R., Colavitti, R., Palazzotti, B., Borrello, S., et al. (2000). Deregulated manganese superoxide dismutase expression and resistance to oxidative injury in p53-deficient cells. Cancer Research, 60, 4654–4660.
Forrester, K., Ambs, S., Lupold, S. E., Kapust, R. B., Spillare, E. A., Weinberg, W. C., et al. (1996). Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proceedings of the National Academy of Sciences of the United States of America, 93, 2442–2447.
Ambs, S., Merriam, W. G., Ogunfusika, M. O., Bennett, W. P., Ishibe, N., Hussain, S. P., et al. (1998). p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nature Medicine, 4, 1371–1376.
Ambs, S., Bennett, W. P., Merriam, W. G., Ogunfusika, M. O., Oser, S. M., Harrington, A. M., et al. (1999). Relationship between p53 mutations and inducible nitric oxide synthase expression in human colorectal cancer. Journal of the National Cancer Institute, 91, 86–88.
Kops, G. J., Dansen, T. B., Polderman, P. E., Saarloos, I., Wirtz, K. W., Coffer, P. J., et al. (2002). Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature, 419, 316–321.
Park, H. J., Carr, J. R., Wang, Z., Nogueira, V., Hay, N., Tyner, A. L., et al. (2009). FoxM1, a critical regulator of oxidative stress during oncogenesis. EMBO Journal, 28, 2908–2918.
Tanaka, H., Matsumura, I., Ezoe, S., Satoh, Y., Sakamaki, T., Albanese, C., et al. (2002). E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kappaB activity that facilitates MnSOD-mediated ROS elimination. Molecular Cell, 9, 1017–1029.
Dolado, I., Swat, A., Ajenjo, N., De, V. G., Cuadrado, A., & Nebreda, A. R. (2007). p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell, 11, 191–205.
Kennedy, N. J., Cellurale, C., & Davis, R. J. (2007). A radical role for p38 MAPK in tumor initiation. Cancer Cell, 11, 101–103.
Bulavin, D. V., Phillips, C., Nannenga, B., Timofeev, O., Donehower, L. A., Anderson, C. W., et al. (2004). Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf) pathway. Nature Genetics, 36, 343–350.
Helzlsouer, K. J., Selmin, O., Huang, H. Y., Strickland, P. T., Hoffman, S., Alberg, A. J., et al. (1998). Association between glutathione S-transferase M1, P1, and T1 genetic polymorphisms and development of breast cancer. Journal of the National Cancer Institute, 90, 512–518.
Havre, P. A., O'Reilly, S., McCormick, J. J., & Brash, D. E. (2002). Transformed and tumor-derived human cells exhibit preferential sensitivity to the thiol antioxidants. N-acetyl cysteine and penicillamine. Cancer Research, 62, 1443–1449.
Polyak, K., Xia, Y., Zweier, J. L., Kinzler, K. W., & Vogelstein, B. (1997). A model for p53-induced apoptosis. Nature, 389, 300–305.
Johnson, T. M., Yu, Z. X., Ferrans, V. J., Lowenstein, R. A., & Finkel, T. (1996). Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proceedings of the National Academy of Sciences of the United States of America, 93, 11848–11852.
Drane, P., Bravard, A., Bouvard, V., & May, E. (2001). Reciprocal down-regulation of p53 and SOD2 gene expression-implication in p53 mediated apoptosis. Oncogene, 20, 430–439.
Dhar, S. K., Xu, Y., Chen, Y., & St Clair, D. K. (2006). Specificity protein 1-dependent p53-mediated suppression of human manganese superoxide dismutase gene expression. Journal of Biological Chemistry, 281, 21698–21709.
Pani, G., Colavitti, R., Bedogni, B., Fusco, S., Ferraro, D., Borrello, S., et al. (2004). Mitochondrial superoxide dismutase: a promising target for new anticancer therapies. Current Medicinal Chemistry, 11, 1299–1308.
Ito, K., Hirao, A., Arai, F., Matsuoka, S., Takubo, K., Hamaguchi, I., et al. (2004). Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature, 431, 997–1002.
Diehn, M., Cho, R. W., Lobo, N. A., Kalisky, T., Dorie, M. J., Kulp, A. N., et al. (2009). Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature, 458, 780–783.
Smith, J., Ladi, E., Mayer-Proschel, M., & Noble, M. (2000). Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proceedings of the National Academy of Sciences of the United States of America, 97, 10032–10037.
Jang, Y. Y., & Sharkis, S. J. (2007). A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood, 110, 3056–3063.
Ito, K., Hirao, A., Arai, F., Takubo, K., Matsuoka, S., Miyamoto, K., et al. (2006). Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature Medicine, 12, 446–451.
Tothova, Z., Kollipara, R., Huntly, B. J., Lee, B. H., Castrillon, D. H., Cullen, D. E., et al. (2007). FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell, 128, 325–339.
Chen, C., Liu, Y., Liu, R., Ikenoue, T., Guan, K. L., Liu, Y., et al. (2008). TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. Journal of Experimental Medicine, 205, 2397–2408.
Ranganathan, A. C., Adam, A. P., & Aguirre-Ghiso, J. A. (2006). Opposing roles of mitogenic and stress signaling pathways in the induction of cancer dormancy. Cell Cycle, 5, 1799–1807.
Adam, A. P., George, A., Schewe, D., Bragado, P., Iglesias, B. V., Ranganathan, A. C., et al. (2009). Computational identification of a p38SAPK-regulated transcription factor network required for tumor cell quiescence. Cancer Research, 69, 5664–5672.
Schewe, D. M., & Aguirre-Ghiso, J. A. (2008). ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proceedings of the National Academy of Sciences of the United States of America, 105, 10519–10524.
Schafer, Z. T., Grassian, A. R., Song, L., Jiang, Z., Gerhart-Hines, Z., Irie, H. Y., et al. (2009). Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature, 461, 109–113.
Kuo, W., Lin, J., & Tang, T. K. (2000). Human glucose-6-phosphate dehydrogenase (G6PD) gene transforms NIH 3T3 cells and induces tumors in nude mice. International Journal of Cancer, 85, 857–864.
The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. (1994). The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. New England Journal of Medicine, 330, 1029–1035.
Omenn, G. S., Goodman, G. E., Thornquist, M. D., Balmes, J., Cullen, M. R., Glass, A., et al. (1996). Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. New England Journal of Medicine, 334, 1150–1155.
Shackelford, R. E., Heinloth, A. N., Heard, S. C., & Paules, R. S. (2005). Cellular and molecular targets of protein S-glutathiolation. Antioxidants Redox Signaling, 7, 940–950.
Coussens, L. M., & Werb, Z. (2002). Inflammation and cancer. Nature, 420, 860–867.
Schafer, M., & Werner, S. (2008). Cancer as an overhealing wound: an old hypothesis revisited. Nature Reviews Molecular Cell Biology, 9, 628–638.
Phinney, D. G., & Prockop, D. J. (2007). Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells, 25, 2896–2902.
Ishii, T., Itoh, K., Takahashi, S., Sato, H., Yanagawa, T., Katoh, Y., et al. (2000). Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. Journal of Biological Chemistry, 275, 16023–16029.
Huang, C., Han, Y., Wang, Y., Sun, X., Yan, S., Yeh, E. T., et al. (2009). SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO Journal, 28, 2748–2762.
Scortegagna, M., Ding, K., Oktay, Y., Gaur, A., Thurmond, F., Yan, L. J., et al. (2003). Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nature Genetics, 35, 331–340.
Conrotto, P., Corso, S., Gamberini, S., Comoglio, P. M., & Giordano, S. (2004). Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene, 23, 5131–5137.
Fischer, O. M., Giordano, S., Comoglio, P. M., & Ullrich, A. (2004). Reactive oxygen species mediate Met receptor transactivation by G protein-coupled receptors and the epidermal growth factor receptor in human carcinoma cells. Journal of Biological Chemistry, 279, 28970–28978.
Klarmann, G. J., Hurt, E. M., Mathews, L. A., Zhang, X., Duhagon, M. A., Mistree, T., et al. (2009). Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clinical & Experimental Metastasis, 26, 433–446.
O'Dell, T. J., Hawkins, R. D., Kandel, E. R., & Arancio, O. (1991). Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proceedings of the National Academy of Sciences of the United States of America, 88, 11285–11289.
Niethammer, P., Grabher, C., Look, A. T., & Mitchison, T. J. (2009). A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature, 459, 996–999.
Taylor, B. L., Rebbapragada, A., & Johnson, M. S. (2001). The FAD-PAS domain as a sensor for behavioral responses in Escherichia coli. Antioxidants Redox Signaling, 3, 867–879.
Graeber, T. G., Osmanian, C., Jacks, T., Housman, D. E., Koch, C. J., Lowe, S. W., et al. (1996). Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature, 379, 88–91.
Hentze, M. W., & Kuhn, L. C. (1996). Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proceedings of the National Academy of Sciences of the United States of America, 93, 8175–8182.
Acknowledgments
The authors wish to thank Prof. Lido Calorini, as like as members of their laboratories for suggestions and insightful discussions. Authors are supported by the Italian Association for Cancer Research (AIRC) (grant# IG 8634 to G.P. and grant # IG8797 to P.C.), by Istituto Toscano Tumori and Regione Toscana (TRESOR grants and PorCreo grant to P. C.).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pani, G., Galeotti, T. & Chiarugi, P. Metastasis: cancer cell’s escape from oxidative stress. Cancer Metastasis Rev 29, 351–378 (2010). https://doi.org/10.1007/s10555-010-9225-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-010-9225-4