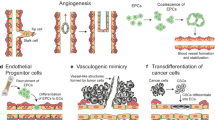
Abstract
Angiogenesis is an important mediator of tumor progression. As tumors expand, diffusion distances from the existing vascular supply increases resulting in hypoxia. Sustained expansion of a tumor mass requires new blood vessel formation to provide rapidly proliferating tumor cells with an adequate supply of oxygen and metabolites. The key regulator of hypoxia-induced angiogenesis is the transcription factor hypoxia inducible factor (HIF)-1. Multiple HIF-1 target genes have been shown to modulate angiogenesis by promoting the mitogenic and migratory activities of endothelial cells. Because of this, hypoxia-induced angiogenesis has become an attractive target for cancer therapy, however the mechanisms involved during this process and how best to target it for cancer therapy are still under investigation. This review will cover the current understanding of hypoxia-induced tumor angiogenesis and discuss the caveats of hypoxia-targeted antiangiogenic therapy for the treatment of cancer.
Similar content being viewed by others
References
Folkman, J. (1971). Tumor angiogenesis: therapeutic implications. New England Journal of Medicine, 285, 1182–1186.
Gimbrone, M. A., Jr., Leapman, S. B., Cotran, R. S., & Folkman, J. (1972). Tumor dormancy in vivo by prevention of neovascularization. Journal of Experimental Medicine, 136, 261–276.
Brem, S., Brem, H., Folkman, J., Finkelstein, D., & Patz, A. (1976). Prolonged tumor dormancy by prevention of neovascularization in the vitreous. Cancer Research, 36, 2807–2812.
Parangi, S., O’Reilly, M., Christofori, G., Holmgren, L., Grosfeld, J., Folkman, J. et al. (1996). Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proceedings of the National Academy of Sciences of the United States of America, 93, 2002–2007.
Holmgren, L., O’Reilly, M. S., Folkman, J. (1995). Dormancy of micrometastases: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nature Medicine, 1, 149–153.
Carmeliet, P. (2000). Mechanisms of angiogenesis and arteriogenesis. Nature Medicine, 6, 389–395.
Bergers, G., & Benjamin, L. E. (2003). Tumorigenesis and the angiogenic switch. Nature Reviews Cancer, 3, 401–410.
Naumov, G. N., Akslen, L. A., & Folkman, J. (2006). Role of angiogenesis in human tumor dormancy: Animal models of the angiogenic switch. Cell Cycle, 5, 1779–1787.
Folkman, J., Watson, K., Ingber, D., & Hanahan, D. (1989). Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature, 339, 58–61.
North, S., Moenner, M., & Bikfalvi, A. (2005). Recent developments in the regulation of the angiogenic switch by cellular stress factors in tumors. Cancer Letter, 218, 1–14.
Lin. E. Y., Li, J. F., Gnatovskiy, L., Deng, Y., Zhu, L., Grzesik, D. A., et al. (2006). Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Research, 66, 11238–11246.
Nozawa, H., Chiu, C., & Hanahan, D. (2006). Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America, 103, 12493–12498.
Holash, J., Maisonpierre, P. C., Compton. D., Boland, P., Alexander, C. R., Zagzag, D., et al. (1999). Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science, 284, 1994–1998.
Giordano, F. J., & Johnson, R. S (2001). Angiogenesis: The role of the microenvironment in flipping the switch. Current Opinion in Genetics & Development, 11, 35–40.
Semenza, G. L. (2004). Hydroxylation of HIF-1: Oxygen sensing at the molecular level. Physiology (Bethesda), 19, 176–182.
Peng, J., Zhang, L., Drysdale, L., & Fong, G. H. (2000). The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proceedings of the National Academy of Sciences of the United States of America, 97, 8386–8391.
Ema, M., Taya, S., Yokotani, N., Sogawa, K., Matsuda, Y., & Fujii-Kuriyama, Y. (1997). A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proceedings of the National Academy of Sciences of the United States of America, 94, 4273–4278.
Makino, Y., Cao, R., Svensson, K., Bertilsson, G., Asman, M., Tanaka, H., et al. (2001). Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature, 414, 550–554.
Ryan, H. E., Lo, J., & Johnson, R. S. (1998). HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO Journal, 17, 3005–3015.
Maxwell, P. H., Dachs, G. U., Gleadle, J. M., Nicholls, L. G., Harris, A. L., Stratford, I. J., et al. (1997). Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proceedings of the National Academy of Sciences of the United States of America, 94, 8104–8109.
Stoeltzing, O., McCarty, M. F., Wey, J. S., Fan, F., Liu, W., Belcheva, A., et al. (2004) Ellis LM: Role of hypoxia-inducible factor 1alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. Journal of the National Cancer Institute, 96, 946–956.
Jensen, R. L., Ragel, B. T., Whang, K., & Gillespie, D. (2006). Inhibition of hypoxia inducible factor-1alpha (HIF-1alpha) decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. Journal of Neuro-oncology, 78, 233–247.
Bos, R., Zhong, H., Hanrahan, C. F., Mommers, E. C., Semenza, G. L., Pinedo, H. M., et al. (2001) Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. Journal of the National Cancer Institute, 93, 309–314
Liao, D., Corle, C., Seagroves, T. N., & Johnson, R. S. (2007). Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Research, 67, 563–572.
Tang, N., Wang, L., Esko, J., Giordano, F. J., Huang, Y., Gerber, H. P., et al. (2004). Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell, 6, 485–495.
Kondo, K., & Kaelin, W. G., Jr. (2001) The von Hippel-Lindau tumor suppressor gene. Experimental Cell Research, 264, 117–125.
Mahon, P. C., Hirota, K., & Semenza, G. L. (2001). FIH-1: A novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes & Development, 15, 2675–2686.
Lando, D., Peet, D. J., Gorman, J. J., Whelan, D. A., Whitelaw, M. L., & Bruick, R. K. (1996). FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes & Development, 16, 1466–1471.
Arany, Z., Huang, L. E., Eckner, R., Bhattacharya, S., Jiang, C., Goldberg, M. A., et al. (1996). An essential role for p300/CBP in the cellular response to hypoxia. Proceedings of the National Academy of Sciences of the United States of America, 93, 12969–12973.
Richard, D. E., Berra, E., Gothie, E., Roux, D., & Pouyssegur, J. (1999). p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. Journal of Biological Chemistry, 274, 32631–32637.
Mylonis, I., Chachami, G., Samiotaki, M., Panayotou, G., Paraskeva, E., Kalousi, A., et al. (2006). Identification of MAPK phosphorylation sites and their role in the localization and activity of hypoxia-inducible factor-1alpha. Journal of Biological Chemistry, 281, 33095–33106.
Shaw, R. J., & Cantley, L. C. (2006). Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature, 441, 424–430.
Zhong, H., Chiles, K., Feldser, D., Laughner, E., Hanrahan, C., Georgescu, M. M., et al. (2000) Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Research, 60, 1541–1545.
Blancher, C., Moore, J. W., Robertson, N., & Harris, A. L. (2001) Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3′-kinase/Akt signaling pathway. Cancer Research, 61, 7349–7355.
Poulaki, V., Mitsiades, C. S., McMullan, C., Sykoutri, D., Fanourakis, G., Kotoula, V., et al. (2003). Regulation of vascular endothelial growth factor expression by insulin-like growth factor I in thyroid carcinomas. Journal of Clinical Endocrinology and Metabolism, 88, 5392–5398.
Zundel, W., Schindler, C., Haas-Kogan, D., Koong, A., Kaper, F., Chen, E., et al. (2000). Loss of PTEN facilitates HIF-1-mediated gene expression. Genes & Development, 14, 391–396.
Grunstein, J., Masbad, J. J., Hickey, R., Giordano, F., & Johnson, R. S. (2000) Isoforms of vascular endothelial growth factor act in a coordinate fashion To recruit and expand tumor vasculature. Molecular and Cellular Biology, 20, 7282–7291.
Forsythe, J. A., Jiang, B. H., Iyer, N. V., Agani, F., Leung, S. W., Koos, R. D., et al. (1996). Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and Cellular Biology, 16, 4604–4613.
Olsson, A. K., Dimberg, A., Kreuger, J., & Claesson-Welsh, L. (2006). VEGF receptor signalling—in control of vascular function. Nature Reviews Molecular Cell Biology, 7, 359–371.
Ferrara, N., Carver-Moore, K., Chen, H., Dowd, M., Lu, L., O’Shea, K. S., et al. (1996). Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature, 380, 439–442.
Grunstein, J., Roberts, W. G., Mathieu-Costello, O., Hanahan, D., Johnson, R. S. (1999). Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Research, 59, 1592–1598.
Mancuso, M. R., Davis, R., Norberg, S. M., O’Brien, S., Sennino, B., Nakahara, T. et al. (2006). Rapid vascular regrowth in tumors after reversal of VEGF inhibition. Journal of Clinical Investigation, 116, 2610–2621.
Das, B., Yeger, H., Tsuchida, R., Torkin, R., Gee, M. F., Thorner, P. S. et al. (2005). A hypoxia-driven vascular endothelial growth factor/Flt1 autocrine loop interacts with hypoxia-inducible factor-1alpha through mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 pathway in neuroblastoma. Cancer Research, 65, 7267–7275.
Morbidelli, L., Donnini, S., & Ziche, M. (2003). Role of nitric oxide in the modulation of angiogenesis. Current Pharmaceutical Design, 9, 521–530.
Kimura, H., Weisz, A., Kurashima, Y., Hashimoto, K., Ogura, T., D’Acquisto, F., et al. (2000). Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: Control of hypoxia-inducible factor-1 activity by nitric oxide. Blood, 95, 189–197.
Palmer, L. A., Semenza, G. L., Stoler, M. H., & Johns, R. A. (1998). Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. American Journal of Physiology, 274, L212–219.
Jung, F., Palmer, L. A., Zhou, N., & Johns, R. A. (2000). Hypoxic regulation of inducible nitric oxide synthase via hypoxia inducible factor-1 in cardiac myocytes. Circulation Research, 86, 319–325.
Coulet, F., Nadaud, S., Agrapart, M., & Soubrier, F. (2003). Identification of hypoxia-response element in the human endothelial nitric oxide synthase gene promoter. Journal of Biological Chemistry, 278, 46230–46240.
Cooke, J. P. (2003). NO and angiogenesis. Atherosclerosis. Supplement, 4, 53–60.
Rossig, L., Fichtlscherer, B., Breitschopf, K., Haendeler, J., Zeiher, A. M., Mulsch, A., et al. (1999). Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. Journal of Biological Chemistry, 274, 6823–6826.
Ellies, L. G, Fishman, M., Hardison, J., Kleeman, J., Maglione, J. E., Manner, C. K. et al. (2003). Mammary tumor latency is increased in mice lacking the inducible nitric oxide synthase. International Journal of Cancer, 106, 1–7.
Cullis, E. R., Kalber, T. L., Ashton, S. E., Cartwright, J. E., Griffiths, J. R., Ryan, A. J., et al. (2006). Tumour overexpression of inducible nitric oxide synthase (iNOS) increases angiogenesis and may modulate the anti-tumour effects of the vascular disrupting agent ZD6126. Microvascular Research, 71, 76–84.
Grose, R., & Dickson, C. (2005). Fibroblast growth factor signaling in tumorigenesis. Cytokine & Growth Factor Reviews, 16, 179–186.
Shi, Y. H., Wang, Y. X., Bingle, L., Gong, L. H., Heng, W. J., Li, Y., et al. (2005). In vitro study of HIF-1 activation and VEGF release by bFGF in the T47D breast cancer cell line under normoxic conditions: involvement of PI-3K/Akt and MEK1/ERK pathways. Journal of Pathology, 205, 530–536.
Pore, N., Liu, S., Haas-Kogan, D. A., O’Rourke, D. M., & Maity, A. (2003). PTEN mutation and epidermal growth factor receptor activation regulate vascular endothelial growth factor (VEGF) mRNA expression in human glioblastoma cells by transactivating the proximal VEGF promoter. Cancer Research, 63, 236–241.
Calvani, M., Rapisarda, A., Uranchimeg, B., Shoemaker. R. H., & Melillo, G. (2006). Hypoxic induction of an HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood, 107, 2705–2712.
Li, J., Shworak, N. W., & Simons, M. (2002). Increased responsiveness of hypoxic endothelial cells to FGF2 is mediated by HIF-1alpha-dependent regulation of enzymes involved in synthesis of heparan sulfate FGF2-binding sites. Journal of Cell Science, 115, 1951–1959.
Brat, D. J., & Mapstone, T. B. (2003). Malignant glioma physiology: Cellular response to hypoxia and its role in tumor progression. Annals of Internal Medicine, 138, 659–668.
Arteaga, C. L. (2002). Epidermal growth factor receptor dependence in human tumors: more than just expression? Oncologist, 7(Suppl 4), 31–39.
Ueda, S., Basaki, Y., Yoshie, M., Ogawa, K., Sakisaka, S., Kuwano, M., et al. (2006). PTEN/Akt signaling through epidermal growth factor receptor is prerequisite for angiogenesis by hepatocellular carcinoma cells that is susceptible to inhibition by gefitinib. Cancer Research, 66, 5346–5353.
Amin, D. N., Hida, K., Bielenberg, D. R., & Klagsbrun, M. (2006). Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Research, 66, 2173–2180.
Yoshida, D., Kim, K., Noha, M., & Teramoto, A. (2006). Hypoxia inducible factor 1-alpha regulates of platelet derived growth factor-B in human glioblastoma cells. Journal of Neuro-oncology, 76, 13–21.
Nakamura, K., Taguchi, E., Miura, T., Yamamoto, A., Takahashi, K., Bichat, F., et al. (2006). KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties. Cancer Research, 66, 9134–9142.
Lederle, W., Stark, H. J., Skobe, M., Fusenig, N. E., & Mueller, M. M. (2006). Platelet-derived growth factor-BB controls epithelial tumor phenotype by differential growth factor regulation in stromal cells. American Journal of Pathology, 169, 1767–1783.
Pages, G., & Pouyssegur, J. (2005). Transcriptional regulation of the Vascular Endothelial Growth Factor gene–a concert of activating factors. Cardiovascular Research, 65, 564–573.
Huang, S., Pettaway, C. A., Uehara, H., Bucana, C. D., & Fidler, I. J. (2001). Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene, 20, 4188–4197.
Schmidt, D., Textor, B., Pein, O. T., Licht, A. H., Andrecht, S., Sator-Schmitt, et al. (2007). Critical role for NF-kappaB-induced JunB in VEGF regulation and tumor angiogenesis. EMBO Journal, 26, 710–719.
Mizukami, Y., Li, J., Zhang, X., Zimmer, M. A., Iliopoulos, O., & Chung, D. C. (2004). Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Research, 64, 1765–1772.
Mizukami, Y., Jo, W. S., Duerr, E. M., Gala, M., Li, J., Zhang, X., et al. (2005) Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nature Medicine, 11, 992–997.
Semenza, G. L. (2003). Targeting HIF-1 for cancer therapy. Nature Reviews Cancer, 3, 721–732.
Fukumura, D., Xavier, R., Sugiura, T., Chen, Y., Park, E. C., Lu N., Selig, M., et al. (1998). Tumor induction of VEGF promoter activity in stromal cells. Cell, 94, 715–725.
Blouw, B., Song, H., Tihan, T., Bosze, J., Ferrara, N., Gerber, H. P., et al. (2003). The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell, 4, 133–146.
Zaman, K., Ryu, H., Hall, D., O’Donovan, K., Lin, K. I., Miller M. P., et al. (1999). Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. Journal of Neuroscience, 19, 9821–9830.
Siddiq, A., Ayoub, I. A., Chavez, J. C., Aminova, L., Shah, S., LaManna, J. C., et al. (2005). Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. Journal of Biological Chemistry, 280, 41732–41743.
Lee, H. J., Kim, K. S., Park, I. H., & Kim, S. U. (2007). Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS ONE 2: e156.
Oosthuyse, B., Moons, L., Storkebaum, E., Beck, H., Nuyens, D., Brusselmans, K., et al. (2001). Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nature Genetics, 28, 131–138
Martinez, P., Esbrit, P., Rodrigo, A., Alvarez-Arroyo, M. V., Martinez, M. E. (2002). Age-related changes in parathyroid hormone-related protein and vascular endothelial growth factor in human osteoblastic cells. Osteoporosis International, 13, 874–881.
Thebaud, B., Ladha, F., Michelakis, E. D., Sawicka, M., Thurston, G., Eaton, F., et al. (2005). Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation, 112, 2477–2486.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liao, D., Johnson, R.S. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev 26, 281–290 (2007). https://doi.org/10.1007/s10555-007-9066-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-007-9066-y