Abstract
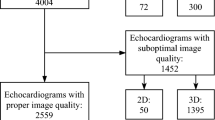
Variability related to image acquisition and interpretation is an important issue of echocardiography in clinical trials. Nevertheless, there is no broadly accepted standard method for quality assessment of echocardiography in clinical research reports. We present analyses based on the echocardiography quality-assurance program of the ongoing STAAB cohort study (characteristics and course of heart failure stages A–B and determinants of progression). In 43 healthy individuals (mean age 50 ± 14 years; 18 females), duplicate echocardiography scans were acquired and mutually interpreted by one of three trained sonographers and an EACVI certified physician, respectively. Acquisition (AcV), interpretation (InV), and inter-observer variability (IOV; i.e., variability between the acquisition-interpretation sequences of two different observers), were determined for selected M-mode, B-mode, and Doppler parameters. We calculated Bland–Altman upper 95% limits of absolute differences, implying that 95% of measurement differences were smaller/equal to the given value: e.g. LV end-diastolic volume (mL): 25.0, 25.0, 27.9; septal e′ velocity (cm/s): 3.03, 1.25, 3.58. Further, 90, 85, and 80% upper limits of absolute differences were determined for the respective parameters. Both, acquisition and interpretation, independently and sizably contributed to IOV. As such, separate assessment of AcV and InV is likely to aid in echocardiography training and quality-assurance. Our results further suggest to routinely determine IOV in clinical trials as a comprehensive measure of imaging quality. The derived 95, 90, 85, and 80% upper limits of absolute differences are suggested as reproducibility targets of future studies, thus contributing to the international efforts of standardization in quality-assurance.

Similar content being viewed by others
Abbreviations
- 2D:
-
Two-dimensional
- A:
-
A-wave velocity
- a′:
-
Peak late- diastolic lengthening velocity
- AcV:
-
Acquisition variability
- CW:
-
Continuous wave
- E:
-
E-wave velocity
- e′:
-
Peak early diastolic lengthening velocity
- EACVI:
-
European Association of Cardiovascular Imaging
- EF:
-
Ejection fraction
- IaInV:
-
Intra-interpretation variability
- ICC:
-
Intraclass correlation coefficient
- InV:
-
Interpretation variability
- IOV:
-
Inter-observer variability
- STAAB:
-
Characteristics and course of heart failure stages A–B and determinants of progression cohort study
- LA:
-
Left atrium
- LV:
-
Left ventricle
- LOA:
-
Limits of agreement
- LVDed:
-
Left ventricular end-diastolic diameter
- LVVed:
-
Left ventricular end-diastolic volume
- LVVes:
-
Left ventricular end-systolic volume
- MAPSE:
-
Mitral annular plane systolic excursion
- PW:
-
Pulsed wave
- RA:
-
Right atrium
- RV:
-
Right ventricle
- s′:
-
Peak systolic shortening velocity
- TAPSE:
-
Tricuspid annular plane systolic excursion
- TDI:
-
Tissue Doppler imaging
References
Crowley AL, Yow E, Barnhart HX, Daubert MA, Bigelow R, Sullivan DC, Pencina M, Douglas PS (2016) Critical review of current approaches for echocardiographic reproducibility and reliability assessment in clinical research. J Am Soc Echocardiogr 29(12):1144–1154.e7. https://doi.org/10.1016/j.echo.2016.08.006
Pellikka PA, Douglas PS, Miller JG, Abraham TP, Baumann R, Buxton DB, Byrd BF 3rd, Chen P, Cook NL, Gardin JM, Hansen G, Houle HC, Husson S, Kaul S, Klein AL, Lang RM, Leong-Poi H, Lopez H, Mahmoud TM, Maslak S, McCulloch ML, Metz S, Nagueh SF, Pearlman AS, Pibarot P, Picard MH, Porter TR, Prater D, Rodriguez R, Sarano ME, Scherrer-Crosbie M, Shirali GS, Sinusas A, Slosky JJ, Sugeng L, Tatpati A, Villanueva FS, von Ramm OT, Weissman NJ, Zamani S (2013) American society of echocardiography cardiovascular technology and research summit: a roadmap for 2020. J Am Soc Echocardiogr 26(4):325–338. https://doi.org/10.1016/j.echo.2013.02.003
Kardys I, Deckers JW, Stricker BH, Vletter WB, Hofman A, Witteman J (2010) Distribution of echocardiographic parameters and their associations with cardiovascular risk factors in the Rotterdam Study. Eur J Epidemiol 25(7):481–490. https://doi.org/10.1007/s10654-010-9453-5
Kou S, Caballero L, Dulgheru R, Voilliot D, De Sousa C, Kacharava G, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez De Diego JJ, Hagendorff A, Henri C, Hristova K, Lopez T, Magne J, De La Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, Salustri A, Van De Veire N, Von Bardeleben RS, Vinereanu D, Voigt JU, Zamorano JL, Donal E, Lang RM, Badano LP, Lancellotti P (2014) Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging 15(6):680–690. https://doi.org/10.1093/ehjci/jet284
Jenkins C, Bricknell K, Hanekom L, Marwick TH (2004) Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J Am Coll Cardiol 44(4):878–886. https://doi.org/10.1016/j.jacc.2004.05.050
Wagner M, Tiffe T, Morbach C, Gelbrich G, Stork S, Heuschmann PU, Consortium S (2016) Characteristics and course of heart failure stages A-B and determinants of progression—design and rationale of the STAAB cohort study. Eur J Prev Cardiol 24(5):468–479. https://doi.org/10.1177/2047487316680693
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16(3):233–270. https://doi.org/10.1093/ehjci/jev014
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8(2):135–160
Thorstensen A, Dalen H, Amundsen BH, Aase SA, Stoylen A (2010) Reproducibility in echocardiographic assessment of the left ventricular global and regional function, the HUNT study. Eur J Echocardiogr 11(2):149–156. https://doi.org/10.1093/ejechocard/jep188
Stritzke J, Linsel-Nitschke P, Markus MR, Mayer B, Lieb W, Luchner A, Doring A, Koenig W, Keil U, Hense HW, Schunkert H, Investigators MK (2009) Association between degenerative aortic valve disease and long-term exposure to cardiovascular risk factors: results of the longitudinal population-based KORA/MONICA survey. Eur Heart J 30(16):2044–2053. https://doi.org/10.1093/eurheartj/ehp287
Colan SD, Shirali G, Margossian R, Gallagher D, Altmann K, Canter C, Chen S, Golding F, Radojewski E, Camitta M, Carboni M, Rychik J, Stylianou M, Tani LY, Selamet Tierney ES, Wang Y, Sleeper LA, Pediatric Heart Network I (2012) The ventricular volume variability study of the Pediatric Heart Network: study design and impact of beat averaging and variable type on the reproducibility of echocardiographic measurements in children with chronic dilated cardiomyopathy. J Am Soc Echocardiogr 25(8):842–854.e6. https://doi.org/10.1016/j.echo.2012.05.004
Otterstad JE, Froeland G, St John Sutton M, Holme I (1997) Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J 18(3):507–513
Barnhart HX, Yow E, Crowley AL, Daubert MA, Rabineau D, Bigelow R, Pencina M, Douglas PS (2014) Choice of agreement indices for assessing and improving measurement reproducibility in a core laboratory setting. Stat Methods Med Res 25(6):2939–2958. https://doi.org/10.1177/0962280214534651
Acknowledgements
We thank Martina Bauer, lead sonographer of the Comprehensive Heart Failure Center (CHFC), for excellent echocardiography teaching, as well as Jasmin Simon, Martin Cramer and Frances Knobeloch, CHFC sonographers, for their valuable contribution to this study.
Funding
The study is supported by the German Ministry of Research and Education within the Comprehensive Heart Failure Centre Würzburg (BMBF 01EO1004 and 01EO1504). This publication was funded by the German Research Foundation (DFG) and the University of Wuerzburg in the funding programme Open Access Publishing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CM reports a co-investigatorship in a trial sponsored by NOVARTIS, a travel grant from THERMO FISHER and a speaker honorarium from AMGEN. GG reports grants from University Göttingen within the FIND-AF-randomized trial (FIND-AF-randomized is supported by an unrestricted grant to the University Göttingen from Boehringer-Ingelheim), grants from University Göttingen within DZHK analysis projects (the DZHK is funded by a BMBF grant), personal fees from Charité - Universitätsmedizin Berlin within the TIM-HF-II trial for data safety and monitoring board membership (TIM-HF-II is supported by a BMBF grant to the Charité), grants from University Hospital Würzburg within the MOOD-HF trial for biometry and steering committee membership (MOOD-HF is supported by a BMBF grant and an unrestricted grant to the University Hospital Würzburg from Lundbeck), outside the submitted work. PUH reports research grants from the German Ministry of Research and Education, European Union, Charité, Berlin Chamber of Physicians, German Parkinson Society, University Hospital Würzburg, Robert-Koch-Institute, German Heart Foundation, Charité–Universitätsmedizin Berlin (within MonDAFIS; MonDAFIS is supported by an unrestricted research grant to the Charité from Bayer), University Göttingen (within FIND-AF-randomized; FIND-AF randomized is supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim), and University Hospital Heidelberg (within RASUNOA-prime; RASUNOA-prime is supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, BMS, Boehringer-Ingelheim, Daiichi Sankyo), outside submitted work. SS reports research grants from the German Ministry of Education and Research, European Union, University Hospital Würzburg; participation in Data Safety Monitoring and Event Adjudication Boards in trials sponsored by ROCHE and MEDTRONIC; principal investigator in trials (co-)sponsored by BOEHRINGER, NOVARTIS, BAYER, LUNDBECK; speaker honaria by BOEHRINGER, SERVIER, NOVARTIS, ASTRA-ZENECA, PFIZER, BAYER. MW declares that he has no conflict of interest. TT declares that she has no conflict of interest. MB declares that she has no conflict of interest.
Ethical approval
The STAAB cohort study protocol and procedures received positive votes from the Ethics Committee of the Medical Faculty (vote 98/13) as well as from the data protection officer of the University of Würzburg (J-117.605-09/13). All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All participants provided written informed consent prior to any study examination.
Rights and permissions
About this article
Cite this article
Morbach, C., Gelbrich, G., Breunig, M. et al. Impact of acquisition and interpretation on total inter-observer variability in echocardiography: results from the quality assurance program of the STAAB cohort study. Int J Cardiovasc Imaging 34, 1057–1065 (2018). https://doi.org/10.1007/s10554-018-1315-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-018-1315-3