Abstract
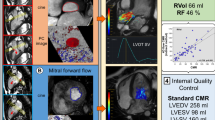
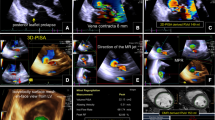
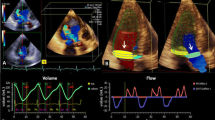
To test the feasibility of assessing mitral regurgitation (MR) severity using cardiac magnetic resonance (CMR) 4D velocity vectors to quantify regurgitant volume (RVol) by analysis of the proximal flow convergence, compared to Doppler based proximal isovelocity surface area (PISA) and CMR volume-based methods. In a prospectively designed study, 27 patients with various grades of MR underwent CMR and echo-Doppler on the same day. By CMR, multiple slices were obtained parallel to the mitral valve by phase-contrast imaging, using 3D velocity vectors, as well as short-axis cine images for left and right ventricular volume measurements. Using dedicated software developed in our laboratory, the perimeter of the proximal flow convergence region was semi-automatically measured for each temporal phase, and for each short-axis slice. The CMR-PISA RVol was calculated as the sum of PISA perimeters throughout systole, multiplied by slice width. For comparison, CMR-volumetric RVol was calculated by 2 methods: Volumetric (difference between left and right ventricular stroke volumes) and Flow-based (stroke volume -aortic flow). Echo-PISA RVol was calculated by echo-Doppler based PISA method. RVol by CMR-PISA correlated highly with echo-PISA (r = 0.87) and with CMR-volumetric (r = 0.86) and CMR-flow (r = 0.72). For comparison Doppler-RVol and CMR-volume-based RVol had r = 0.83. On average CMR-PISA was 16 ± 25 ml less than echo-PISA, but 12 ± 22 ml larger than CMR-volumetric RVol. The observed 3D shape of the PISA envelope by 4D-CMR resembled a hemiellipsoid rather than a hemisphere. This feasibility study suggests that CMR-based 4D-PISA may be able to assess MR severity quantitatively without any geometric assumptions.







Similar content being viewed by others
References
Levine RA, Hagege AA, Judge DP, Padala M, Dal-Bianco JP, Aikawa E, Beaudoin J, Bischoff J, Bouatia-Naji N, Bruneval P, Butcher JT, Carpentier A, Chaput M, Chester AH, Clusel C, Delling FN, Dietz HC, Dina C, Durst R, Fernandez-Friera L, Handschumacher MD, Jensen MO, Jeunemaitre XP, Le Marec H, Le Tourneau T, Markwald RR, Merot J, Messas E, Milan DP, Neri T, Norris RA, Peal D, Perrocheau M, Probst V, Puceat M, Rosenthal N, Solis J, Schott JJ, Schwammenthal E, Slaugenhaupt SA, Song JK, Yacoub MH (2015) Mitral valve disease–morphology and mechanisms. Nat Rev Cardiol 12(12):689–710. https://doi.org/10.1038/nrcardio.2015.161
Enriquez-Sarano M, Akins CW, Vahanian A (2009) Mitral regurgitation. Lancet 373(9672):1382–1394. https://doi.org/10.1016/S0140-6736(09)60692-9
Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ (2003) Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 16(7):777–802. https://doi.org/10.1016/S0894-7317(03)00335-3
Enriquez-Sarano M, Seward JB, Bailey KR, Tajik AJ (1994) Effective regurgitant orifice area: a noninvasive Doppler development of an old hemodynamic concept. J Am Coll Cardiol 23 (2):443–451
Sechtem U, Pflugfelder PW, Cassidy MM, White RD, Cheitlin MD, Schiller NB, Higgins CB (1988) Mitral or aortic regurgitation: quantification of regurgitant volumes with cine MR imaging. Radiology 167(2):425–430. https://doi.org/10.1148/radiology.167.2.3357951
Kon MW, Myerson SG, Moat NE, Pennell DJ (2004) Quantification of regurgitant fraction in mitral regurgitation by cardiovascular magnetic resonance: comparison of techniques. J Heart Valve Dis 13(4):600–607
Buchner S, Debl K, Poschenrieder F, Feuerbach S, Riegger GA, Luchner A, Djavidani B (2008) Cardiovascular magnetic resonance for direct assessment of anatomic regurgitant orifice in mitral regurgitation. Circ Cardiovasc Imaging 1(2):148–155. https://doi.org/10.1161/CIRCIMAGING.107.753103
Moreno FL, Hagan AD, Holmen JR, Pryor TA, Strickland RD, Castle CH (1984) Evaluation of size and dynamics of the inferior vena cava as an index of right-sided cardiac function. Am J Cardiol 53(4):579–585
Hodnett PA, Glielmi CB, Davarpanah AH, Scanlon TG, Ward E, Collins JD, Weale PJ, Carr JC (2012) Inline directionally independent peak velocity evaluation reduces error in peak antegrade velocity estimation in patients referred for cardiac valvular assessment. AJR 198(2):344–350
Matsumura Y, Saracino G, Sugioka K, Tran H, Greenberg NL, Wada N, Toyono M, Fukuda S, Hozumi T, Thomas JD, Yoshikawa J, Yoshiyama M, Shiota T (2008) Determination of regurgitant orifice area with the use of a new three-dimensional flow convergence geometric assumption in functional mitral regurgitation. J Am Soc Echocardiogr 21(11):1251–1256. https://doi.org/10.1016/j.echo.2008.09.004
Schmidt FP, Gniewosz T, Jabs A, Munzel T, Hink U, Lancellotti P, von Bardeleben RS (2014) Usefulness of 3D-PISA as compared to guideline endorsed parameters for mitral regurgitation quantification. Int J Cardiovasc Imaging 30(8):1501–1508. https://doi.org/10.1007/s10554-014-0496-7
Thavendiranathan P, Liu S, Datta S, Rajagopalan S, Ryan T, Igo SR, Jackson MS, Little SH, De Michelis N, Vannan MA (2013) Quantification of chronic functional mitral regurgitation by automated 3-dimensional peak and integrated proximal isovelocity surface area and stroke volume techniques using real-time 3-dimensional volume color Doppler echocardiography: in vitro and clinical validation. Circ Cardiovasc Imaging 6(1):125–133. https://doi.org/10.1161/CIRCIMAGING.112.980383
Yosefy C, Levine RA, Solis J, Vaturi M, Handschumacher MD, Hung J (2007) Proximal flow convergence region as assessed by real-time 3-dimensional echocardiography: challenging the hemispheric assumption. J Am Soc Echocardiogr 20(4):389–396. https://doi.org/10.1016/j.echo.2006.09.006
Krieger EV, Lee J, Branch KR, Hamilton-Craig C (2016) Quantitation of mitral regurgitation with cardiac magnetic resonance imaging: a systematic review. Heart 102(23):1864–1870. https://doi.org/10.1136/heartjnl-2015-309054
Suinesiaputra A, Bluemke DA, Cowan BR, Friedrich MG, Kramer CM, Kwong R, Plein S, Schulz-Menger J, Westenberg JJ, Young AA, Nagel E (2015) Quantification of LV function and mass by cardiovascular magnetic resonance: multi-center variability and consensus contours. J Cardiovasc Magn Reson 17:63. https://doi.org/10.1186/s12968-015-0170-9
Chai P, Mohiaddin R (2005) How we perform cardiovascular magnetic resonance flow assessment using phase-contrast velocity mapping. J Cardiovasc Magn Reson 7(4):705–716
Myerson SG, d’Arcy J, Christiansen JP, Dobson LE, Mohiaddin R, Francis JM, Prendergast B, Greenwood JP, Karamitsos TD, Neubauer S (2016) Determination of clinical outcome in mitral regurgitation with cardiovascular magnetic resonance quantification. Circulation 133(23):2287–2296. https://doi.org/10.1161/CIRCULATIONAHA.115.017888
Uretsky S, Gillam L, Lang R, Chaudhry FA, Argulian E, Supariwala A, Gurram S, Jain K, Subero M, Jang JJ, Cohen R, Wolff SD (2015) Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: a prospective multicenter trial. J Am Coll Cardiol 65(11):1078–1088. https://doi.org/10.1016/j.jacc.2014.12.047
Aplin M, Kyhl K, Bjerre J, Ihlemann N, Greenwood JP, Plein S, Uddin A, Tonder N, Host NB, Ahlstrom MG, Hove J, Hassager C, Iversen K, Vejlstrup NG, Lav Madsen P (2016) Cardiac remodelling and function with primary mitral valve insufficiency studied by magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 17(8):863–870. https://doi.org/10.1093/ehjci/jev321
Dyverfeldt P, Bissell M, Barker AJ, Bolger AF, Carlhall CJ, Ebbers T, Francios CJ, Frydrychowicz A, Geiger J, Giese D, Hope MD, Kilner PJ, Kozerke S, Myerson S, Neubauer S, Wieben O, Markl M (2015) 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Res 17:72. https://doi.org/10.1186/s12968-015-0174-5
Funding
The study was funded by the Atkins Medical Research Fund, a research grant provided by the Technion-Israel Institute of Technology for “Interdisciplinary Cooperation of the Medical Faculty with the Faculty of Electrical Engineering”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Ethics committee approval was given by the Rambam Helsinki Committee (RMB-11-0282).
Informed consent
All patients signed informed consent before entering the study.
Appendix: The Algorithm for finding the 4D PISA and calculation of the RVol
Appendix: The Algorithm for finding the 4D PISA and calculation of the RVol
The calculation is based on finding the surface area of an isovelocity 3D shape. This area is found by using isovelocity lines to create connected 3D shapes. For each slice-location, the perimeters of each isovelocity region are calculated and integrated over time. In contrast to PISA using ultrasound, there is no geometric assumption concerning the isovelocity 3D shape, which is derived from the isovelocity line in each slice-location, and use of 3D flow vectors means the flow measurements are not limited by the direction of the scanning plane.
This method is based on three main steps: (1) find the suitable areas in which the blood flows, (2) connect between areas to create 3D shapes, and finally (3) calculate the RVol for each shape.
In contrast to Doppler methods where the velocity threshold is determined as the aliasing velocity used in capturing the image, in this method, the velocity threshold can be determined after scanning and in fact, we repeat calculations for multiple velocity thresholds (from 15 to 35 cm/s), in order to investigate the influence of the threshold on the result and to determine the optimal threshold.
For this method, one must first manually mark the region of the regurgitant flow by means of an elliptical ROI (Fig. 1 left), and specify the slices and time-stamps in which this flow appeared. This process was performed from the cine sequences and flow sequences taken both perpendicular to the MR jet and in the direction of the jet.
Find suitable areas
In this step, the voxels in each velocity-image that meet the given criteria are marked to create connected regions within the image. A minimal number of voxels are required to account as a region of interest in order to remove small areas, which are assumed to be measurement noises.
For each time-stamp and slice-location of the selected time-stamps and slices, all the voxels within the elliptic ROI which have an absolute velocity below the isovelocity threshold in the required direction are marked. Image smoothing is then performed by applying dilation and erosion. Then, the regions are separated into connected areas (Fig. 1 middle).
In order to simplify calculations and account for noisy irregular shapes, we chose to approximate each region as a matching ellipse, using least squares fitting (Fig. 1 right). The least squares fitting method is based on the voxels in the perimeter of the region. The least squares fitting algorithm provides 5 parameters of the ellipse: The center according to the X axis, the center according to the Y axis, the major radius, the minor radius and the rotation angle. In some cases, the matched ellipse expands outside the original marked ROI. For those cases, the center of the ellipse and the ellipses’ radii were changed in a way that most of the ellipse (at least 80%) will be inside the original marked area. This is done by iteratively changing the ellipse’s center to be the center of the voxels of the ellipse within the ROI and the major radius is multiplied by the ratio between the number of pixels of the ellipse area within the ROI and the area of the ellipse. The perimeter for ellipses is calculated using the elliptic integral.
Find connected 3D shapes
The regions were assigned with 3D-shape-indices, where the index is equal for regions that belongs to the same 3D-shape. Neighbouring slices or neighbouring time-stamps (for each slice) are checked for the presence of connected areas between them. A connected area is defined as an area where the centre of mass is within the area of the neighbouring shape (Fig. 2).
Calculate MR volume for each 3D-shape
After the separation to 3D-shape-indices is completed, the flux through each 3D-shape is calculated as the surface area, multiplied by the velocity threshold. To obtain the RVol, for each phase, the flux is multiplied by the time between phases, and finally volumes for all phases throughout systole are summed to obtain total RVol. Surface area is calculated for each slice as the perimeter multiplied by slice thickness. Surface areas for all slices are summed to obtain the surface area of the shape. From those volumes, the maximal volume is selected.
Selection of velocity threshold
As described earlier, the velocity threshold is selected in the post-processing procedure and therefore the PISA using CMR method can be used with varying velocities. Hence instead of selecting one velocity threshold and computing the volume using that threshold, one can calculate RVol separately for a number of velocities and then select the optimal velocity threshold. After examing the data, we chose to select the maximal RVol. Graphs of RVol as a function of velocity threshold are shown in Fig. 8. For most cases where the MR is significant the RVol is fairly constant, however for lower grades of MR, the RVol often falls linearly with increasing velocities, probably a partial volume effect.
Rights and permissions
About this article
Cite this article
Gorodisky, L., Agmon, Y., Porat, M. et al. Assessment of mitral regurgitation by 3-dimensional proximal flow convergence using magnetic resonance imaging: comparison with echo-Doppler. Int J Cardiovasc Imaging 34, 793–802 (2018). https://doi.org/10.1007/s10554-017-1290-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-017-1290-0