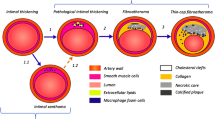
Abstract
Many apparent healthy persons die from cardiovascular disease, despite major advances in prevention and treatment of cardiovascular disease. Traditional cardiovascular risk factors are able to predict cardiovascular events in the long run, but fail to assess current disease activity or nearby cardiovascular events. There is a clear relation between the occurrence of cardiovascular events and the presence of so-called vulnerable plaques. These vulnerable plaques are characterized by active inflammation, a thin cap and a large lipid pool. Spectroscopy is an optical imaging technique which depicts the interaction between light and tissues, and thereby shows the biochemical composition of tissues. In recent years, impressive advances have been made in spectroscopy technology and intravascular spectroscopy is able to assess the composition of plaques of interest and thereby to identify and actually quantify plaque vulnerability. This review summarizes the current evidence for spectroscopy as a measure of plaque vulnerability and discusses the potential role of intravascular spectroscopic imaging techniques.



Similar content being viewed by others
References
Lindholm LH, Mendis S (2007) Prevention of cardiovascular disease in developing countries. Lancet 370(9589):720–722
Naghavi M, Libby P, Falk E et al (2003) From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 108(14):1664–1672
Manoharan G, Ntalianis A, Muller O et al (2009) Severity of coronary stenoses responsible for acute coronary syndromes. Am J Cardiol 103(9):1138–1188
Fayad ZA, Fuster V (2001) Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ Res 89(4):305–316
Pearson TA (2002) New tools for coronary risk assessment: what are their advantages and limitations? Circulation 105(7):886–892
Hamdan A, Assali A, Fuchs S et al (2007) Imaging of vulnerable coronary artery plaques. Catheter Cardiovasc Interv 70(1):65–74
Chang R (1971) Basic principles of spectroscopy. McGraw-Hill, New York
Kolodgie FD, Burke AP, Farb A et al (2001) The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol 16(5):285–292
Shah PK (2007) Molecular mechanisms of plaque instability. Curr Opin Lipidol 18(5):492–499
Shah PK (2003) Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol 41(4 Suppl S):15S–22S
Johnson JL (2007) Matrix metalloproteinases: influence on smooth muscle cells and atherosclerotic plaque stability. Expert Rev Cardiovasc Ther 5(2):265–282
Cipollone F, Fazia M, Mincione G et al (2004) Increased expression of transforming growth factor-beta1 as a stabilizing factor in human atherosclerotic plaques. Stroke 35(10):2253–2257
Fleiner M, Kummer M, Mirlacher M et al (2004) Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation 110(18):2843–2850
Salenius JP, Brennan JF III, Miller A et al (1998) Biochemical composition of human peripheral arteries examined with near-infrared Raman spectroscopy. J Vasc Surg 27(4):710–719
van de Poll SW, Romer TJ, Puppels GJ et al (2002) Imaging of atherosclerosis. Raman spectroscopy of atherosclerosis. J Cardiovasc Risk 9(5):255–261
Romer TJ, Brennan JF III, Fitzmaurice M et al (1998) Histopathology of human coronary atherosclerosis by quantifying its chemical composition with Raman spectroscopy. Circulation 97(9):878–885
Romer TJ, Brennan JF III, Puppels GJ et al (2000) Intravascular ultrasound combined with Raman spectroscopy to localize and quantify cholesterol and calcium salts in atherosclerotic coronary arteries. Arterioscler Thromb Vasc Biol 20(2):478–483
van de Poll SW, Romer TJ, Volger OL et al (2001) Raman spectroscopic evaluation of the effects of diet and lipid-lowering therapy on atherosclerotic plaque development in mice. Arterioscler Thromb Vasc Biol 21(10):1630–1635
Scepanovic OR, Fitzmaurice M, Gardecki JA et al (2006) Detection of morphological markers of vulnerable atherosclerotic plaque using multimodal spectroscopy. J Biomed Opt 11(2):021007
Motz JT, Hunter M, Galindo LH et al (2004) Optical fiber probe for biomedical Raman spectroscopy. Appl Opt 43(3):542–554
Buschman HP, Marple ET, Wach ML et al (2000) In vivo determination of the molecular composition of artery wall by intravascular Raman spectroscopy. Anal Chem 72(16):3771–3775
Motz JT, Fitzmaurice M, Miller A et al (2006) In vivo Raman spectral pathology of human atherosclerosis and vulnerable plaque. J Biomed Opt 11(2):021003
Virmani R, Kolodgie FD, Burke AP et al (2000) Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 20(5):1262–1275
Cassis LA, Lodder RA (1993) Near-IR imaging of atheromas in living arterial tissue. Anal Chem 65(9):1247–1256
Dempsey RJ, Cassis LA, Davis DG, Lodder RA (1997) Near-infrared imaging and spectroscopy in stroke research: lipoprotein distribution and disease. Ann N Y Acad Sci 820:149–169
Jaross W, Neumeister V, Lattke P, Schuh D (1999) Determination of cholesterol in atherosclerotic plaques using near infrared diffuse reflection spectroscopy. Atherosclerosis 147(2):327–337
Moreno PR, Lodder RA, Purushothaman KR et al (2002) Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infrared spectroscopy. Circulation 105(8):923–927
Lilledahl MB, Haugen OA, Barkost M, Svaasand LO (2006) Reflection spectroscopy of atherosclerotic plaque. J Biomed Opt 11(2):021005
Moreno PR, Muller JE (2003) Detection of high-risk atherosclerotic coronary plaques by intravascular spectroscopy. J Interv Cardiol 16(3):243–252
Moreno PR, Muller JE (2003) Detection of high-risk atherosclerotic coronary plaques by intravascular spectroscopy. J Interv Cardiol 16(3):243–252
Waxman S, Ishibashi F, Caplan JD (2007) Rationale and use of near-infrared spectroscopy for detection of lipid-rich and vulnerable plaques. J Nucl Cardiol 14(5):719–728
Hiro T, Leung CY, De Guzman S et al (1997) Are soft echoes really soft? Intravascular ultrasound assessment of mechanical properties in human atherosclerotic tissue. Am Heart J 133(1):1–7
Hatsukami TS, Ross R, Polissar NL, Yuan C (2000) Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 102(9):959–964
Chu B, Ferguson MS, Underhill H et al (2005) Images in cardiovascular medicine. Detection of carotid atherosclerotic plaque ulceration, calcification, and thrombosis by multicontrast weighted magnetic resonance imaging. Circulation 112(1):e3–e4
Finn AV, Joner M, Nakazawa G et al (2007) Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115(18):2435–2441
Joner M, Finn AV, Farb A et al (2006) Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 48(1):193–202
Hong MK, Mintz GS, Lee CW et al (2008) A three-vessel virtual histology intravascular ultrasound analysis of frequency and distribution of thin-cap fibroatheromas in patients with acute coronary syndrome or stable angina pectoris. Am J Cardiol 101(5):568–572
Hong MK, Mintz GS, Lee CW et al (2007) Comparison of virtual histology to intravascular ultrasound of culprit coronary lesions in acute coronary syndrome and target coronary lesions in stable angina pectoris. Am J Cardiol 100(6):953–959
Cutlip DE, Chhabra AG, Baim DS et al (2004) Beyond restenosis: five-year clinical outcomes from second-generation coronary stent trials. Circulation 110(10):1226–1230
Luker GD, Luker KE (2008) Optical imaging: current applications and future directions. J Nucl Med 49(1):1–4
Chen J, Tung CH, Mahmood U et al (2002) In vivo imaging of proteolytic activity in atherosclerosis. Circulation 105(23):2766–2771
Weissleder R, Tung CH, Mahmood U, Bogdanov A Jr (1999) In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol 17(4):375–378
Uchida Y, Nakamura F, Tomaru T, Morita T, Oshima T, Sasaki T, Morizuki S, Hirose J (1995) Prediction of acute coronary syndromes by percutaneous coronary angioscopy in patients with stable angina. Am Heart J 130(2):195–203
Manfrini O, Mont E, Leone O et al (2006) Sources of error and interpretation of plaque morphology by optical coherence tomography. Am J Cardiol 98(2):156–159
Stefanadis C, Toutouzas K, Tsiamis E et al (2001) Thermography of human arterial system by means of new thermography catheters. Catheter Cardiovasc Interv 54(1):51–58
Rudd JH, Davies JR, Weissberg PL (2005) Imaging of atherosclerosis—can we predict plaque rupture? Trends Cardiovasc Med 15(1):17–24
Cordeiro MA, Lima JA (2006) Atherosclerotic plaque characterization by multidetector row computed tomography angiography. J Am Coll Cardiol 47(8 Suppl):C40–C47
Ogawa M, Ishino S, Mukai T et al (2004) (18)F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med 45(7):1245–1250
Conflict of interest
Dr. Andries J. Smit is one of the founders of DiagnOptics B.V., Groningen, the Netherlands, manufacturer of the AGE-Reader, which is a skin autofluorescence method not mentioned in the present article. There are no potential or actual, personal, political, or financial interests by any of the other authors in the material, information, or techniques described in the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bruggink, J.L.M., Meerwaldt, R., van Dam, G.M. et al. Spectroscopy to improve identification of vulnerable plaques in cardiovascular disease. Int J Cardiovasc Imaging 26, 111–119 (2010). https://doi.org/10.1007/s10554-009-9500-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-009-9500-z