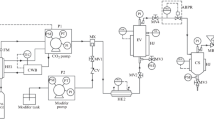
A study was carried out on the precipitation of heavy fractions and the isolation of asphaltenes from a petroleum sample using supercritical carbon dioxide (SC-CO2) as the antisolvent. The experiments were carried out in a laboratory supercritical fluid extraction device using a GAS (gas antisolvent) procedure at from 50 to 140°C and pressure from 10 to 30 MPa. We determined the effects of temperature and pressure as well as of the addition of a hydrocarbon diluent on the yields of the isolated fractions. The elemental and microelement composition was determined as well as the structural properties of the components isolated in the SC-CO2 medium at various temperatures. An increase in temperature at pressures above 20 MPa as well as the addition of small amounts of toluene to the starting petroleum sample gave greater isolation selectivity, greater concentration of asphaltenes in the precipitated fractions, and drier solid particles. In contrast to C7-asphaltenes, CO2-asphaltenes have lower aromaticity, polarity, and metal content. The proposed analytical method permits the isolation of asphaltenes in only a few hours, does not require large volumes of organic solvents, and yields asphaltenes in amounts sufficient for subsequent detailed study of their composition and properties.





Similar content being viewed by others
References
C. Leyva, J. Ancheyta, C. Berrueco, et al., Fuel Processing Technology, 106, 734-738 (2013).
P. Luo and Y. Gu, Fuel, 86, 1069-1078 (2007).
R. G. Santos, W. Loh, A. C. Bannwart, et al., Brazilian Journal of Chemical Engineering, 31, 571-590 (2014).
M. S. Rana, V. Samana, J. Ancheyta, et al., Fuel, 86, 1216-1231 (2007).
P. Luo, X. Wang, and Y. Gu, Fluid Phase Equilibria, 291, 103-110 (2010).
J. Ancheyta, G. Centeno, F. Trejo, et al., Energy & Fuels, 16, 1121-1127 (2002).
H. Alboudwarej, J. Beck, W. Y. Svrcek, et al., Energy & Fuels, 16, 462-469 (2002).
B. Eckermann and A. Vogelpohl, Chem. Eng. Technol., 13, 258-264 (1990).
Z. Liu, G. Y. Yang, Y. Lu, et al., Journal of Supercritical Fluids, 16, 27-31 (1999).
US Patent 0032340 Al.
P. Zanganeh, H. Dashti, and S. Ayatollahi, Fuel, 160, 132-139 (2015).
S. Soroush, B. Breure, T. W. de Loos, et al., Journal of Supercritical Fluids, 94, 59-64 (2014).
T. F. Headen and E. S. Boek, Energy & Fuels, 25, 503-508 (2011).
R. N. Magomedov, A. V. Pripakhaylo, and T. A. Maryutina, Journal of Supercritical Fluids, 119, 150 (2017).
R. N. Cavalcanti and M. A. A. Meireles, in: J. Pawliszyn (editor), Comprehensive Sampling and Sample Preparation, Vol. 2, Elsevier, Amsterdam (2012), pp. 117-133.
Thermophysical Properties for Carbon Dioxide, NIST Chemistry WebBook, SRD 69, URL: https://webbook.nist.gov/chemistry/fluid/
D. Espinat, D. Fenistein, L. Bane, et al., Energy & Fuels, 18, 1243-1249 (2004).
X. Wang and Y. Gu, Energy & Fuels, 25, 5232-5241 (2011).
H. Ni, C. S. Hsu, P. Lee, et al., Fuel, 141, 74-81(2015).
S. Rudyk, P. Spirov, and A. Tyrovolas, Journal of CO 2 Utilization, 24, 471-478 (2018).
S. I. Andersen and J. G. Speight, Petroleum Science and Technology, 19, Nos. 1/2, 1-34 (2001).
M. R. Yakubov, K. O. Sinyashin, G. R. Abilova, et al., Petroleum Chemistry, 57, No. 10, 849-854 (2017).
G. Caumette, C.-P. Lienemann, I. Merdrignac, et al., J. Anal. At. Spectrom., 25, 1123-1129 (2010).
E. Rogel, C. Ovalles, and M. E. Moir, Energy & Fuels, 23, 4515-4521 (2009).
This study was carried out with the support of the Russian Science Fund (Project No. 18-73-00345). The authors express their gratitude the Engineering Center of the Moscow Institute of Physics and Technology for permitting use of scientific equipment in the framework of the Agreement of Science and Technology Cooperation (March 2, 2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 3, pp. 49 – 56, May – June, 2019.
Rights and permissions
About this article
Cite this article
Magomedov, R.N., Pripakhaylo, A.V., Foteeva, L.S. et al. Method for Isolating Asphaltenes from Petroleum by Their Precipitation from Supercritical Carbon Dioxide. Chem Technol Fuels Oils 55, 287–298 (2019). https://doi.org/10.1007/s10553-019-01032-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-019-01032-6