Abstract
Purpose
Tamoxifen is frequently prescribed to prevent breast cancer recurrence. Tamoxifen is a prodrug and requires bioactivation by CYP2D6. Tamoxifen use is often limited by adverse effects including severe hot flashes. There is paucity of prospectively collected data in terms of CYP2D6 genotype and measured tamoxifen, 4-hydroxytamoxifen and endoxifen concentrations in relation to hot flash severity during tamoxifen therapy.
Methods
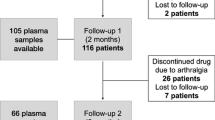
We conducted a longitudinal prospective study of breast cancer patients on tamoxifen (n = 410). At each visit, blood samples were collected, and patients completed a standardized hot flash survey (n = 1144) that reflected hot flash severity during the 7 days prior to the visit. Plasma concentrations of tamoxifen, 4-hydroxytamoxifen, and endoxifen were measured using liquid chromatography-tandem mass spectrometry and genotyping was carried out for CYP2D6. A linear mixed-effects regression analysis assessed the association of covariates in relation to the hot flash severity score (HFSS).
Results
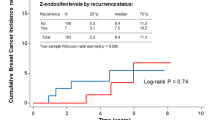
Median age at first assessment was 50 years with 61.9% of patients considered peri-menopausal. Most patients (92.2%) experienced hot flash symptoms with 51.0% having low HFSS (0–4) and 7.32% experiencing HFSS > 25. Age was significantly associated with hot flash severity, with patients aged 45–59 more likely to have higher HFSS. Neither duration of tamoxifen therapy nor observed tamoxifen, endoxifen and 4-hydroxy tamoxifen plasma concentration predicted hot flash severity. Genetic variation in CYP2D6 or CYP3A4 was not predictive of hot flash severity.
Conclusions
Hot flash severity during tamoxifen therapy can not be accounted for by CYP2D6 genotype or observed plasma concentration of tamoxifen, 4-hydroxytamoxifen, or endoxifen.



Similar content being viewed by others
Abbreviations
- HFSS :
-
Hot flash severity score
- SNP :
-
Single-nucleotide polymorphism
- UM, NM, IM, PM :
-
Ultra, normal, intermediate, poor metabolizer
- CYP :
-
Cytochrome P450
- 4-OH-tam :
-
4-hydroxytamoxifen
- NDM-tam :
-
N-desmethyl-tamoxifen
- LC-MS/MS:
-
Liquid chromatography-mass spectrometry
- SSRI :
-
Selective serotonin reuptake inhibitor
- BMI :
-
Body mass index
References
Ahern TP, Hertz DL, Damkier P et al (2017) Cytochrome P-450 2D6 (CYP2D6) Genotype and breast cancer recurrence in tamoxifen-treated patients: evaluating the importance of loss of heterozygosity. Am J Epidemiol 185:75–85
Hawse JR, Subramaniam M, Cicek M et al (2013) Endoxifen’s molecular mechanisms of action are concentration dependent and different than that of other anti-estrogens. PLoS ONE 8:e54613
Lim YC, Desta Z, Flockhart DA, Skaar TC (2005) Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol 55:471–478
Murdter TE, Schroth W, Bacchus-Gerybadze L et al (2011) Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther 89:708–717
Baxter SD, Teft WA, Choi YH, Winquist E, Kim RB (2014) Tamoxifen-associated hot flash severity is inversely correlated with endoxifen concentration and CYP3A4*22. Breast Cancer Res Treat 145:419–428
Zembutsu H (2015) Pharmacogenomics toward personalized tamoxifen therapy for breast cancer. Pharmacogenomics 16:287–296
Sideras K, Ingle JN, Ames MM et al (2010) Coprescription of tamoxifen and medications that inhibit CYP2D6. J Clin Oncol 28:2768–2776
Binkhorst L, Mathijssen RH, van Herk-Sukel MP et al (2013) Unjustified prescribing of CYP2D6 inhibiting SSRIs in women treated with tamoxifen. Breast Cancer ResTreat 139:923–929
Kelly CM, Juurlink DN, Gomes T et al (2010) Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ 340:c693
Madlensky L, Natarajan L, Tchu S et al (2011) Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther 89:718–725
Saladores P, Murdter T, Eccles D et al (2015) Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J 15:84–94
Bhave MA, Henry NL (2017) Extended endocrine therapy: is 5 years enough? Curr Oncol Rep 19:16
Rossmanith WG, Ruebberdt W (2009) What causes hot flushes? The neuroendocrine origin of vasomotor symptoms in the menopause. Gynecol Endocrinol 25:303–314
Del RM, Citi V, Crucitta S et al (2016) Pharmacogenetics of CYP2D6 and tamoxifen therapy: light at the end of the tunnel? Pharmacol Res 107:398–406
Deecher DC, Dorries K (2007) Understanding the pathophysiology of vasomotor symptoms (hot flushes and night sweats) that occur in perimenopause, menopause, and postmenopause life stages. Arch Womens Ment Health 10:247–257
Kronenberg F (2010) Menopausal hot flashes: a review of physiology and biosociocultural perspective on methods of assessment. J Nutr 140:1380S–1385S
Lorizio W, Wu AH, Beattie MS et al (2012) Clinical and biomarker predictors of side effects from tamoxifen. Breast Cancer ResTreat 132:1107–1118
Mortimer JE, Flatt SW, Parker BA et al (2008) Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat 108:421–426
Goetz MP, Rae JM, Suman VJ et al (2005) Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 23:9312–9318
Henry NL, Rae JM, Li L et al (2009) Association between CYP2D6 genotype and tamoxifen-induced hot flashes in a prospective cohort. Breast Cancer ResTreat 117:571–575
Sestak I, Kealy R, Nikoloff M et al (2012) Relationships between CYP2D6 phenotype, breast cancer and hot flushes in women at high risk of breast cancer receiving prophylactic tamoxifen: results from the IBIS-I trial. Br J Cancer 107:230–233
Dezentje VO, Gelderblom H, van Schaik RH et al (2014) CYP2D6 genotype in relation to hot flashes as tamoxifen side effect in a Dutch cohort of the tamoxifen exemestane adjuvant multinational (TEAM) trial. Breast Cancer Res Treat 143:171–179
Fox P, Balleine RL, Lee C et al (2016) Dose escalation of tamoxifen in patients with low endoxifen level: evidence for therapeutic drug monitoring-the TADE study. Clin Cancer Res 22:3164–3171
Jager NG, Koornstra RH, Vincent AD et al (2013) Hot flashes are not predictive for serum concentrations of tamoxifen and its metabolites. BMC Cancer 13:612
Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H (2001) Methodologic lessons learned from hot flash studies. J Clin Oncol 19:4280–4290
R Core Team (2016) R. A language and environment for statistical computing, in R Foundation for Statistical Computing. Vienna, Austria. (online source)
Gupta P, Sturdee DW, Palin SL et al (2006) Menopausal symptoms in women treated for breast cancer: the prevalence and severity of symptoms and their perceived effects on quality of life. Climacteric 9:49–58
Irvin WJ Jr, Walko CM, Weck KE et al (2011) Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: a multicenter study. J Clin Oncol 29:3232–3239
Mortimer J, Behrendt CE (2013) Severe menopausal symptoms are widespread among survivors of breast cancer treatment regardless of time since diagnosis. J Palliat Med 16:1130–1134
Acknowledgements
We would like to thank Cameron Ross and Sara Mansell for their technical support; and Jody Murray for her administrative assistance. We would also like to thank the team at the London Regional Cancer Program. This work was supported by the Wolfe Medical Research Chair in Pharmacogenomics, Canadian Institutes of Health Research, Drug Safety and Effectiveness Network (DSEN-PREVENT, FRN-117588), and the Ontario Research Fund-Research Excellence Grant from the Ontario Ministry of Research and Innovation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in this study were approved by the University of Western Ontario Research Ethics Board and in accordance with the ethical standards of 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all participants.
Rights and permissions
About this article
Cite this article
Jansen, L.E., Teft, W.A., Rose, R.V. et al. CYP2D6 genotype and endoxifen plasma concentration do not predict hot flash severity during tamoxifen therapy. Breast Cancer Res Treat 171, 701–708 (2018). https://doi.org/10.1007/s10549-018-4876-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4876-x