Abstract
Purpose
We aimed to investigate the role of palbociclib, a first-in-class cyclin-dependent kinase 4 and 6 inhibitor, in postmenopausal women with highly pretreated endocrine therapy-resistant metastatic breast cancer (MBC).
Methods
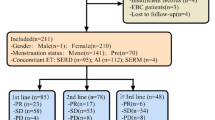
Between 28 September 2015 and 14 March 2017, a compassionate use program was established in the University Hospitals Leuven in which 82 postmenopausal women with estrogen receptor-positive, HER2-negative MBC were included after at least four lines of systemic treatment. The efficacy and safety analysis was performed in 82 patients who had received at least one dose of palbociclib and who had at least 6-month follow-up at the data cut-off point. The primary objective was the evaluation of efficacy of the combination of palbociclib and endocrine therapy with clinical benefit as primary endpoint, defined as the absence of progressive disease and being on treatment for at least 6 months. Secondary objectives were the evaluation of toxicity and the identification of potential predictors for clinical benefit.
Results
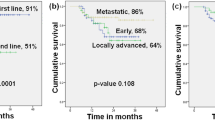
The median age of the patients was 67.1 years (range 34.8–85.9) at the time of inclusion. The average duration of treatment was 5.6 months (range 1–19), with a median progression-free survival of 3.17 (95% CI 2.76–4.70) months. At the data cut-off point, 10 patients were still on treatment with palbociclib. In this highly pretreated setting, 34 patients experienced no progressive disease within 6 months, resulting in an overall clinical benefit rate (CBR) of 41.5%. 20.7% (17/82) showed stable disease for ≥ 9 months and 13.4% for ≥ 12 months. None of the investigated predicting factors were significantly associated with clinical benefit at 6 months. For 43.9% of the patients, treatment delay or dose reduction was indicated.
Conclusions
Palbociclib in combination with endocrine therapy shows an unexpectedly high CBR and favorable safety profile in heavily pretreated endocrine-resistant estrogen receptor-positive, HER2-negative MBC patients.



Similar content being viewed by others
Abbreviations
- MBC:
-
Metastatic breast cancer
- PFS:
-
Progression-free survival
- CBR:
-
Clinical benefit rate
- ER:
-
Estrogen receptor
- CDK:
-
Cyclin-dependent kinases
- CUP:
-
Compassionate use program
- ULN:
-
Upper limit of normal
References
Boér K (2016) Impact of palbociclib combinations on treatment of advanced estrogen receptor-positive/human epidermal growth factor 2-negative breast cancer. Onco Targets Ther 9:6119–6125
Maurer C, Martel S, Zardavas D, Ignatiadis M (2017) New agents for endocrine resistance in breast cancer. The Breast 34:1–11
Almstedt K, Schmidt M (2015) Targeted therapies overcoming endocrine resistance in hormone receptor-positive breast cancer. Breast Care (Basel) 10(3):168–172
Cardoso F, Costa A, Senkus E et al (2017) 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol 28(1):16–33
Augereau P, Patsouris A, Bourbouloux E et al (2017) Hormonoresistance in advanced breast cancer: a new revolution in endocrine therapy. Ther Adv Med Oncol 9(5):335–346
Vidula N, Rugo HS (2016) Cyclin-dependent kinase 4/6 inhibitors for the treatment of breast cancer: a review of preclinical and clinical data. Clin Breast Cancer 16(1):8–17
Turner NC, Ro J, André F et al (2015) Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 373(3):209–219
Finn RS, Crown JP, Lang I et al (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35
Dickler MN, Tolaney SM, Rugo HS et al. (2017) MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2-metastatic breast cancer. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-17-0754
Sledge GW Jr, Toi M, Neven P et al (2017) MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35(25):2875–2884
Bilgin B, Sendur MAN, Şener Dede D, Akıncı MB, Yalçın B (2017) A current and comprehensive review of cyclin-dependent kinase inhibitors for the treatment of metastatic breast cancer. Curr Med Res Opin 33(9):1559–1569
Romero D (2016) Breast cancer: MONALEESA-2 and FALCON—PFS advantage. Nat Rev Clin Oncol 13(12):717
Beaver JA, Amiri-Kordestani L, Charlab R et al (2015) FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res 21(21):4760–4766
Cristofanilli M, Turner NC, Bondarenko I et al (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17(4):425–439
Finn RS, Crown J, Lang I et al. (2017) Overall survival results from the randomized phase II study of palbociclib (P) in combination with letrozole (L) vs letrozole alone for frontline treatment of ER+/HER2-advanced breast cancer (PALOMA-1; TRIO-18). J Clin Oncol 35(no15_suppl): 1001. https://doi.org/10.1200/JCO.2017.35.15_suppl.1001
Finn RS, Martin M, Rugo HS et al (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936
Xu H, Yu S, Liu Q et al (2017) Recent advances of highly selective CDK4/6 inhibitors in breast cancer. J Hematol Oncol 10(1):97
Chirila C, Mitra D, Colosia A et al (2017) Comparison of palbociclib in combination with letrozole or fulvestrant with endocrine therapies for advanced/metastatic breast cancer: network meta-analysis. Curr Med Res Opin 33(8):1457–1466
Patnaik A, Rosen LS, Tolaney SM et al (2016) Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov 6(7):740–753
DeMichele A, Clark AS, Tan KS et al (2015) CDK 4/6 inhibitor palbociclib (PD0332991) in Rb + advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res 21(5):995–1001
Finn RS, Dering J, Conklin D et al (2009) PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 11:R77
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Iwase H, Yamamoto Y (2015) Clinical benefit of sequential use of endocrine therapies for metastatic breast cancer. Int. J Clin Oncol 20(2):253–261
Kim ES, Scott LJ. Palbociclib (2017) A review in HR-positive, HER2-negative, advanced or metastatic breast cancer. Target Oncol 12(3):373–383
Hu W, Sung T, Jessen BA et al (2016) Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res 22(8):2000–2008
Rugo HS (2016) Dosing and safety implications for oncologists when administering everolimus to patients with hormone receptor-positive breast cancer. Clin Breast Cancer 16(1):18–22
Fribbens C, O’Leary B, Kilburn L et al (2016) Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol 34(25):2961–2968
Shah AN, Cristofanilli M (2017) The growing role of CDK4/6 inhibitors in treating hormone receptor-positive advanced breast cancer. Curr Treat Options Oncol 18(1):6
O’Leary B, Hrebien S, Morden JP et al (2018) Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun 9(1):896
Acknowledgements
The authors wish to thank Dr. A. Laenen (KULeuven, Belgium) for her help with the statistics. Pfizer is acknowledged for their support with this CUP.
Funding
This study was financially supported by Pfizer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Appendix: Toxicity assessments and criteria for dose reductions, delays, or discontinuation of treatment
Appendix: Toxicity assessments and criteria for dose reductions, delays, or discontinuation of treatment



Rights and permissions
About this article
Cite this article
Hoste, G., Punie, K., Wildiers, H. et al. Palbociclib in highly pretreated metastatic ER-positive HER2-negative breast cancer. Breast Cancer Res Treat 171, 131–141 (2018). https://doi.org/10.1007/s10549-018-4827-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4827-6