Abstract
Purpose
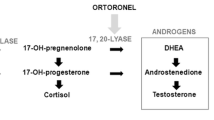
Seviteronel (INO-464) is an oral, selective cytochrome P450c17a (CYP17) 17,20-lyase (lyase) and androgen receptor inhibitor with in vitro and in vivo anti-tumor activity. This open-label phase 1 clinical study evaluated safety, tolerability, pharmacokinetics (PK), and activity of once-daily (QD) seviteronel in women with locally advanced or metastatic TNBC or ER+ breast cancer.
Methods
Seviteronel was administered in de-escalating 750, 600, and 450 mg QD 6-subject cohorts. The 750 mg QD start dose was a phase 2 dose determined for men with castration-resistant prostate cancer in (Shore et al. J Clin Oncol 34, 2016). Enrollment at lower doses was initiated in the presence of dose-limiting toxicities (DLTs). The primary objective of this study was to determine seviteronel safety, tolerability, and MTD. The secondary objectives included description of its PK in women and its initial activity, including clinical benefit rate at 4 (CBR16) and 6 months (CBR24).
Results
Nineteen women were enrolled. A majority of adverse events (AEs) were Grade (Gr) 1/2, independent of relationship; the most common were tremor (42%), nausea (42%), vomiting (37%), and fatigue (37%). Four Gr 3/4 AEs (anemia, delirium, mental status change, and confusional state) deemed possibly related to seviteronel occurred in four subjects. DLTs were observed at 750 mg (Gr 3 confusional state with paranoia) and 600 mg (Gr 3 mental status change and Gr 3 delirium) QD, with none at 450 mg QD. The recommended phase 2 dose (RP2D) was 450 mg QD, and at the RP2D, 4 of 7 subjects reached at least CBR16 (2 TNBC subjects and 2 ER+ subjects achieved CBR16 and CBR24, respectively); no objective tumor responses were reported.
Conclusions
Once-daily seviteronel was generally well tolerated in women with and 450 mg QD was chosen as the RP2D.

Similar content being viewed by others
References
Shore ND, Gupta S, Fleming MT, Berry WR, Zhang J, Kurman MR, Eisner JR, Moore WR (2016) Once-nightly (QD) dual CYP17-Lyase (L) inhibitor / androgen receptor (AR) antagonist VT-464 in patients with CRPC. J Clin Oncol 34 (2): [2016 Genitourinary Cancers Symposium])
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. https://doi.org/10.1038/35021093
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98 (19):10869–10874. https://doi.org/10.1073/pnas.19136709898/19/10869
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100 (14):8418–8423. https://doi.org/10.1073/pnas.09326921000932692100
Soreide JA, Lea OA, Varhaug JE, Skarstein A, Kvinnsland S (1992) Androgen receptors in operable breast cancer: relation to other steroid hormone receptors, correlations to prognostic factors and predictive value for effect of adjuvant tamoxifen treatment. Eur J Surg Oncol 18(2):112–118
Kimura N, Mizokami A, Oonuma T, Sasano H, Nagura H (1993) Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. J Histochem Cytochem 41(5):671–678
Lea OA, Kvinnsland S, Thorsen T (1989) Improved measurement of androgen receptors in human breast cancer. Cancer Res 49(24 Pt 1):7162–7167
Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J, Cameron D, Goldstein D, Duss S, Nicoulaz AL, Brisken C, Fiche M, Delorenzi M, Iggo R (2005) Identification of molecular apocrine breast tumours by microarray analysis. Oncogene 24(29):4660–4671. https://doi.org/10.1038/sj.onc.1208561
Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, Gerald WL (2006) An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 25(28):3994–4008. doi:1209415 [pii] https://doi.org/10.1038/sj.onc.1209415
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121(7):2750–2767. https://doi.org/10.1172/JCI45014
Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, Blackwell K, Rugo H, Nabell L, Forero A, Stearns V, Doane AS, Danso M, Moynahan ME, Momen LF, Gonzalez JM, Akhtar A, Giri DD, Patil S, Feigin KN, Hudis CA, Traina TA, Translational Breast Cancer Research C (2013) Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res 19(19):5505–5512. https://doi.org/10.1158/1078-0432.CCR-12-3327
Bonnefoi H, Grellety T, Tredan O, Saghatchian M, Dalenc F, Mailliez A, L’Haridon T, Cottu P, Abadie-Lacourtoisie S, You B, Mousseau M, Dauba J, Del Piano F, Desmoulins I, Coussy F, Madranges N, Grenier J, Bidard FC, Proudhon C, MacGrogan G, Orsini C, Pulido M, Goncalves A (2016) A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12 – 1). Ann Oncol 27(5):812–818. https://doi.org/10.1093/annonc/mdw067
Traina TA, Miller K, Yardley DA, O’Shaughnessy J, Cortes J, Awada A, Kelly CM, Trudeau ME, Schmid P, Gianni L, Garcia-Estevez L, Nanda R, Ademuyiwa FO, Chan S, Steinberg JL, Blaney ME, Tudor IC, Uppal H, Peterson AC, Hudis CA (2015) Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR + triple-negative breast cancer (TNBC). ASCO Meet Abstr 33 (15_suppl):1003
De Amicis F, Thirugnansampanthan J, Cui Y, Selever J, Beyer A, Parra I, Weigel NL, Herynk MH, Tsimelzon A, Lewis MT, Chamness GC, Hilsenbeck SG, Ando S, Fuqua SA (2010) Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat 121(1):1–11. https://doi.org/10.1007/s10549-009-0436-8
Ciupek A, Rechoum Y, Gu G, Gelsomino L, Beyer AR, Brusco L, Covington KR, Tsimelzon A, Fuqua SA (2015) Androgen receptor promotes tamoxifen agonist activity by activation of EGFR in ERalpha-positive breast cancer. Breast Cancer Res Treat 154(2):225–237. https://doi.org/10.1007/s10549-015-3609-7
Cochrane DR, Bernales S, Jacobsen BM, Cittelly DM, Howe EN, D’Amato NC, Spoelstra NS, Edgerton SM, Jean A, Guerrero J, Gomez F, Medicherla S, Alfaro IE, McCullagh E, Jedlicka P, Torkko KC, Thor AD, Elias AD, Protter AA, Richer JK (2014) Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res 16(1):R7. https://doi.org/10.1186/bcr3599
Rechoum Y, Iacopetta D, Barone I, Rovito D, Ando S, Weigel N, Fuqua SAW (2013) Collaboration of AR and ERα in conferring resistance to an aromatase inhibitor. ASCO Meet Abstr 31:579
Rafferty SW, Eisner JR, Moore WR, Schotzinger RJ, Hoekstra WJ (2014) Highly-selective 4-(1,2,3-triazole)-based P450c17a 17,20-lyase inhibitors. Bioorg Med Chem Lett 24(11):2444–2447. https://doi.org/10.1016/j.bmcl.2014.04.024
Norris JD, Ellison SJ, Baker JG, Stagg DB, Wardell SE, Park S, Alley HM, Baldi RM, Yllanes A, Andreano KJ, Stice JP, Lawrence SA, Eisner JR, Price DK, Moore WR, Figg WD, McDonnell DP (2017) Androgen receptor antagonism drives cytochrome P450 17A1 inhibitor efficacy in prostate cancer. J Clin Invest 127(6):2326–2338. https://doi.org/10.1172/JCI87328
Ellison SJNJ., Wardell S, Eisner JR, Hoekstra WJ, Stagg DB, Alley HM, Moore WR, McDonnell DP (2016) Effects of the dual selective CYP17 lyase inhibitor and androgen receptor (AR) antagonist, VT-464, on AR+ and ER+ tumor models in vitro and in vivo. [abstract]. Cancer Res 76 P3–14
O’Shaughnessy J, Campone M, Brain E, Neven P, Hayes D, Bondarenko I, Griffin TW, Martin J, De Porre P, Kheoh T, Yu MK, Peng W, Johnston S (2016) Abiraterone acetate, exemestane or the combination in postmenopausal patients with estrogen receptor-positive metastatic breast cancer. Ann Oncol 27(1):106–113. https://doi.org/10.1093/annonc/mdv487
Martin RM, Lin CJ, Costa EM, de Oliveira ML, Carrilho A, Villar H, Longui CA, Mendonca BB (2003) P450c17 deficiency in Brazilian patients: biochemical diagnosis through progesterone levels confirmed by CYP17 genotyping. J Clin Endocrinol Metab 88(12):5739–5746. https://doi.org/10.1210/jc.2003-030988
Kok RC, Timmerman MA, Wolffenbuttel KP, Drop SL, de Jong FH (2010) Isolated 17,20-lyase deficiency due to the cytochrome b5 mutation W27X. J Clin Endocrinol Metab 95(3):994–999. https://doi.org/10.1210/jc.2008-1745
Attard G, Reid AH, Auchus RJ, Hughes BA, Cassidy AM, Thompson E, Oommen NB, Folkerd E, Dowsett M, Arlt W, de Bono JS (2012) Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab 97(2):507–516. https://doi.org/10.1210/jc.2011-2189
de Bono J, Pezaro CJ, Gillessen S, Shore ND, Nordquist LT, Efstathiou E, Araujo JC, Berry WR, Liu G, Vogelzang NJ, Omlin AG, Schotzinger RJ, Eisner JR, Moore WR (2015) The oral CYP17-Lyase (L) inhibitor VT-464 in patients with CRPC. J Clin Oncol 33 ((suppl 7)):abstr 187
Charmandari E, Nicolaides NC, Chrousos GP (2014) Adrenal insufficiency. Lancet 383(9935):2152–2167. https://doi.org/10.1016/S0140-6736(13)61684-0
Longo DL FA, Kasper DL, Hauser SL, Jameson J, Loscalzo J (eds) (2012) Harrison’s principles of internal Medicine. 18 edn. McGraw-Hill, New York
Michels A, Michels N (2014) Addison disease: early detection and treatment principles. Am Fam Physician 89(7):563–568
Zidan J, Chetver L, Hussein O, Zucker M (2010) Effect of letrozole on plasma lipids, triglycerides, and estradiol in postmenopausal women with metastatic breast cancer. Oncologist 15(11):1159–1163. https://doi.org/10.1634/theoncologist.2009-0222
Li W, O’Shaughnessy J, Hayes D, Campone M, Bondarenko I, Zbarskaya I, Brain E, Stenina M, Ivanova O, Graas MP, Neven P, Ricci D, Griffin T, Kheoh T, Yu M, Gormley M, Martin J, Schaffer M, Zelinsky K, De Porre P, Johnston SR (2016) Biomarker associations with efficacy of abiraterone acetate and exemestane in postmenopausal patients with estrogen receptor-positive metastatic breast cancer. Clin Cancer Res 22(24):6002–6009. https://doi.org/10.1158/1078-0432.CCR-15-2452
Schwartzberg LS, Yardley DA, Elias AD, Patel M, LoRusso P, Burris HA, Gucalp A, Peterson AC, Blaney ME, Steinberg JL, Gibbons JA, Traina TA (2017) A phase I/Ib study of enzalutamide alone and in combination with endocrine therapies in women with advanced breast cancer. Clin Cancer Res 23(15):4046–4054. https://doi.org/10.1158/1078-0432.CCR-16-2339
Eisner J, Abbott DH, Bird IM, Rafferty SW, Moore WR, Schotzinger RJ (2012) Assessment of steroid hormones upstream of P450c17 (CYP17) in chemically castrate male rhesus monkeys following treatment with the CYP17 inhibitors VT-464 and abiraterone acetate (AA). In: The Endocrine Society’s 94th Annual Meeting and Expo:abstr SAT-266
Ng CHMMI., Rea D et al. Phase I/II study of abiraterone acetate (AA) in estrogen receptor (ER) or androgen receptor (AR) positive metastatic breast cancer (MBC). European Society for Medical Oncology Congress, Vienna, September 28–October 2 2012
Baselga J, Campone M, Piccart M, Burris 3rd HA, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366(6):520–529. https://doi.org/10.1056/NEJMoa1109653
Finn RS, Aleshin A, Slamon DJ (2016) Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 18(1):17. https://doi.org/10.1186/s13058-015-0661-5
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, Andre F, Winer EP, Janni W, Verma S, Conte P, Arteaga CL, Cameron DA, Petrakova K, Hart LL, Villanueva C, Chan A, Jakobsen E, Nusch A, Burdaeva O, Grischke EM, Alba E, Wist E, Marschner N, Favret AM, Yardley D, Bachelot T, Tseng LM, Blau S, Xuan F, Souami F, Miller M, Germa C, Hirawat S, O’Shaughnessy J (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375(18):1738–1748. https://doi.org/10.1056/NEJMoa1609709
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, Koh H, Grischke EM, Frenzel M, Lin Y, Barriga S, Smith IC, Bourayou N, Llombart-Cussac A (2017) MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol:JCO2017737585. https://doi.org/10.1200/JCO.2017.73.7585
Gordon MA, D’Amato NC, Gu H, Babbs B, Wulfkuhle J, Petricoin EF, Gallagher I, Dong T, Torkko K, Liu B, Elias A, Richer JK (2017) Synergy between androgen receptor antagonism and inhibition of mTOR and HER2 in breast cancer. Mol Cancer Ther 16(7):1389–1400. https://doi.org/10.1158/1535-7163.MCT-17-0111
Funding
This study was funded by Innocrin Pharmaceuticals. E.S.B-B and J.R.E are compensated employees of Innocrin Pharmaceuticals. T.A.T. receives compensation as a Steering Committee member for Innocrin Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Aditya Bardia and Ayca Gucalp contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bardia, A., Gucalp, A., DaCosta, N. et al. Phase 1 study of seviteronel, a selective CYP17 lyase and androgen receptor inhibitor, in women with estrogen receptor-positive or triple-negative breast cancer. Breast Cancer Res Treat 171, 111–120 (2018). https://doi.org/10.1007/s10549-018-4813-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4813-z