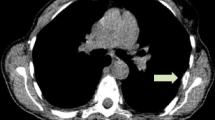
Abstract
Purpose
Premenopausal physiologic steroid levels change cyclically, in contrast to steady state low levels seen in postmenopausal patients. The purpose of this study was to evaluate whether 18F-fluorodeoxyglucose (18F-FDG) uptake in breast cancer is influenced by physiological hormonal fluctuations.
Methods
A total of 160 primary invasive breast cancers from 155 females (54 premenopausal, 101 postmenopausal) who underwent 18F-FDG positron emission tomography/computed tomography before therapy were retrospectively analyzed. The maximal standardized uptake values (SUVmax) of tumors were compared with menstrual phases and menopausal status according to the following subgroups: ‘luminal A-like,’ ‘luminal B-like,’ and ‘non-luminal.’ Additionally, the effect of estradiol (E2) on 18F-FDG uptake in breast cancer cells was evaluated in vitro.
Results
Among premenopausal patients, SUVmax during the periovulatory-luteal phase was significantly higher than that during the follicular phase in luminal A-like tumors (n = 25, p = 0.004), while it did not differ between the follicular phase and the periovulatory-luteal phase in luminal B-like (n = 24) and non-luminal tumors (n = 7). Multiple regression analysis showed menstrual phase, tumor size, and Ki-67 index are independent predictors for SUVmax in premenopausal luminal A-like tumors. There were no significant differences in SUVmax between pre- and postmenopausal patients in any of the subgroups. In in vitro studies, uptake in estrogen receptor-positive cells was significantly augmented when E2 concentration was increased from 0.01 to ≥ 1 nM.
Conclusions
Our data suggest that 18F-FDG uptake may be impacted by physiological hormonal fluctuations during menstrual cycle in luminal A-like cancers, and that E2 could be partly responsible for these events.





Similar content being viewed by others
References
Zasadny KR, Wahl R (1993) Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-d-glucose: variations with body weight and a method for correction. Radiology 189:847–850
Yager JD, Davidson NE (2006) Estrogen carcinogenesis in breast cancer. N Engl J Med 35:270–282
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24:2206–2223
Ko BH, Paik JY, Jung KH, Lee KH (2010) 17beta-estradiol augments 18F-FDG uptake and glycolysis of T47D breast cancer cells via membrane-initiated rapid PI3K–Akt activation. J Nucl Med 51:1740–1747
Neeman M, Degani H (1989) Early estrogen-induced metabolic changes and their inhibition by actinomycin D and cycloheximide in human breast cancer cells: 31P and 13C NMR studies. Proc Natl Acad Sci USA 86:5585–5589
Rivenzon-Segal D, Boldin-Adamsky S, Seger D, Seger R, Degani H (2003) Glycolysis and glucose transporter 1 as markers of response to hormonal therapy in breast cancer. Int J Cancer 107:177–182
Medina RA, Meneses AM, Vera JC, Guzman C, Nualart F, Astuya A et al (2003) Estrogen and progesterone up-regulate glucose transporter expression in ZR-75-1 human breast cancer cells. Endocrinology 144:4527–4535
Dehdashti F, Flanagan FL, Mortimer JE, Katzenellenbogen JA, Welch MJ, Siegel BA (1999) Positron emission tomographic assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy. Eur J Nucl Med 26:51–56
Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ (2001) Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol 19:2797–2803
Riggs BL, Hartmann LC (2003) Selective estrogen-receptor modulators: mechanisms of action and application to clinical practice. N Engl J Med 348:618–629
Dehdashti F, Mortimer JE, Trinkaus K, Naughton MJ, Ellis M, Katzenellenbogen JA et al (2009) PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Res Treat 113:509–517
Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C et al (2005) Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst 97:755–765
Endogenous Hormones and Breast Cancer Collaborative Group, Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ et al (2013) Sex hormones and breast cancer risk in premenopausal women: a collaborative reanalysis of seven prospective studies. Lancet Oncol 14:1009–1019
Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A et al (1999) Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab 84:4025–4030
Holliday DL, Speirs V (2011) Choosing the right cell line for breast cancer research. Breast Cancer Res 13:215
Kuhl CK, Bieling HB, Gieseke J, Kreft BP, Sommer T, Lutterbey G et al (1997) Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology 203:137–144
Lehman CD, Mahoney MC, Newell MS, Bailey L, Barke LD, D’Orsi CJ et al (2014) ACR practice parameter for the performance of contrast-enhanced magnetic resonance imaging (MRI) of the breast. In: Diagnostic radiology: magnetic resonance imaging (MRI) practice parameters and technical standards. American College of Radiology. http://www.acr.org/Quality-Safety/Standards-Guidelines/Practice-Guidelines-by-Modality/MRI. Accessed 1 July 2015
Mann RM, Kuhl CK, Kinkel K, Boetes C (2008) Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol 18:1307–1318
Williams G, Anderson E, Howell A, Watson R, Coyne J, Roberts SA et al (1991) Oral contraceptive (OCP) use increases proliferation and decreases oestrogen receptor content of epithelial cells in the normal human breast. Int J Cancer 48:206–210
Going JJ, Anderson TJ, Battersby S, MacIntyre CC (1988) Proliferative and secretory activity in human breast during natural and artificial menstrual cycles. Am J Pathol 130:193–204
Silva JS, Georgiade GS, Dilley WG, McCarty KS Sr, Wells SA Jr, McCarty KS Jr (1983) Menstrual cycle-dependent variations of breast cyst fluid proteins and sex steroid receptors in the normal human breast. Cancer 51:1297–1302
Pujol P, Daures JP, Thezenas S, Guilleux F, Rouanet P, Grenier J (1998) Changing estrogen and progesterone receptor patterns in breast carcinoma during the menstrual cycle and menopause. Cancer 83:698–705
Smyth CM, Benn DE, Reeve TS (1988) Influence of the menstrual cycle on the concentrations of estrogen and progesterone receptors in primary breast cancer biopsies. Breast Cancer Res Treat 11:45–50
Weimer DA, Donegan WL (1987) Changes in estrogen and progesterone receptor content of primary breast carcinoma during the menstrual cycle. Breast Cancer Res Treat 10:273–278
Horimoto Y, Arakawa A, Tanabe M, Kuroda K, Matsuoka J, Igari F et al (2015) Menstrual cycle could affect Ki67 expression in estrogen receptor-positive breast cancer patients. J Clin Pathol 68:825–829
Lin CY, Ding HJ, Liu CS, Chen YK, Lin CC, Kao CH (2007) Correlation between the intensity of breast FDG uptake and menstrual cycle. Acad Radiol 14:940–944
Khodr ZG, Sherman ME, Pfeiffer RM, Gierach GL, Brinton LA, Falk RT et al (2014) Circulating sex hormones and terminal duct lobular unit involution of the normal breast. Cancer Epidemiol Biomark Prev 23:2765–2773
Thys-Jacobs S, McMahon D, Bilezikian JP (2008) Differences in free estradiol and sex hormone-binding globulin in women with and without premenstrual dysphoric disorder. J Clin Endocrinol Metab 93:96–102
Gil-Rendo A, Martínez-Regueira F, Zornoza G, García-Velloso MJ, Beorlegui C, Rodriguez-Spiteri N (2009) Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg 96:166–170
Shimoda W, Hayashi M, Murakami K, Oyama T, Sunagawa M (2007) The relationship between FDG uptake in PET scans and biological behavior in breast cancer. Breast Cancer 14:260–268
Miyake KK, Nakamoto Y, Kanao S, Tanaka S, Sugie T, Mikami Y et al (2014) Journal Club: diagnostic value of (18)F-FDG PET/CT and MRI in predicting the clinicopathologic subtypes of invasive breast cancer. AJR 203:272–279
Randolph JF Jr, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ (2004) Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab 89:1555–1561
Acknowledgements
The authors thank Hiroyuki Kimura, Ph.D. (Division of Molecular Imaging, Radioisotope Research Center, Kyoto University, and Department of Analytical and Bioinorganic Chemistry, Kyoto Pharmaceutical University) for his great assistance in the in vitro radioisotope experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This project was performed in compliance with the relevant ethical standards in Japan.
Rights and permissions
About this article
Cite this article
Miyake, K.K., Nakamoto, Y., Saji, S. et al. Impact of physiological hormonal fluctuations on 18F-fluorodeoxyglucose uptake in breast cancer. Breast Cancer Res Treat 169, 437–446 (2018). https://doi.org/10.1007/s10549-018-4711-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4711-4