Abstract
Purpose
Results from clinical trials of adjuvant dose-dense chemotherapy in patients with breast cancer are inconsistent.
Methods
A systematic search of MEDLINE identified studies comparing the efficacy of dose-dense adjuvant chemotherapy to a standard treatment. The primary analysis included studies that used identical regimens in the experimental and control groups, but varied only dose density. A secondary analysis included studies that used either different drugs or doses in the experimental and the control groups. Hazard ratios (HRs) and 95% confidence intervals were computed for disease-free survival (DFS) and overall survival (OS) and pooled in a meta-analysis. Subgroup analyses and meta-regression explored drug schedules utilized in control groups and the influence of clinicopathologic variables on benefit from dose-dense therapy.
Results
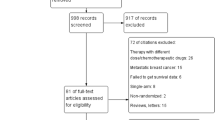
The primary analysis included 5 studies comprising 9819 patients while the secondary analysis included 6 studies comprising 9679 patients. Dose-dense treatment significantly improved DFS (HR 0.85, p < 0.001) and OS (HR 0.86, p = 0.008) in the primary analysis. Similar results were observed in the secondary analysis. Dose-dense schedule was important primarily in studies utilizing paclitaxel every 3 weeks as the control group (interaction p = 0.04 for DFS interaction p = 0.001 for OS). A significantly greater relative magnitude of benefit was observed in pre-menopausal women and those with nodal involvement, but there was no influence of hormone receptor status on results.
Conclusions
Adjuvant dose-dense regimens improve breast cancer outcomes. It remains uncertain whether the observed benefit reflects the impact of dose density or the inferiority of paclitaxel every 3 weeks as a control group.



Similar content being viewed by others
Abbreviations
- CALGB:
-
Cancer and Leukemia Group B
- CI:
-
Confidence intervals
- DFS:
-
Disease-free survival
- ECOG:
-
Eastern Cooperative Oncology Group
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hazard ratio
- OS:
-
Overall survival
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M et al (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet 379:432–444
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF et al (2015) Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373(21):2005–2014. https://doi.org/10.1056/NEJMoa1510764
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
De Laurentiis M, Cancello G, D’Agostino D, Giuliano M, Giordano A, Montagna E et al (2008) Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol 26:44–53
Sparano JA, Zhao F, Martino S, Ligibel JA, Perez EA, Saphner T et al (2015) Long-term follow-up of the E1199 phase III trial evaluating the role of taxane and schedule in operable breast cancer. J Clin Oncol 33:2353–2360
Norton L (1997) Evolving concepts in the systemic drug therapy of breast cancer. Semin Oncol 24(4 Suppl 10):3–10
Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ et al (2003) Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21:1431–1439
Cameron D, Morden JP, Canney P, Velikova G, Coleman R, Bartlett J et al (2017) Accelerated versus standard epirubicin followed by cyclophosphamide, methotrexate, and fluorouracil or capecitabine as adjuvant therapy for breast cancer in the randomised UK TACT2 trial (CRUK/05/19): a multicentre, phase 3, open-label, randomised controlled trial. Lancet Oncol 18:929–945
Baldini E, Gardin G, Giannessi PG, Evangelista G, Roncella M, Prochilo T et al (2003) Accelerated versus standard cyclophosphamide, epirubicin and 5-fluorouracil or cyclophosphamide, methotrexate and 5-fluorouracil: a randomized phase III trial in locally advanced breast cancer. Ann Oncol 14:227–232
Venturini M, Del Mastro L, Aitini E, Baldini E, Caroti C, Contu A et al (2005) Dose-dense adjuvant chemotherapy in early breast cancer patients: results from a randomized trial. J Natl Cancer Inst 97:1724–1733
Foukakis T, von Minckwitz G, Bengtsson NO, Brandberg Y, Wallberg B, Fornander T et al (2016) Effect of tailored dose-dense chemotherapy vs standard 3-weekly adjuvant chemotherapy on recurrence-free survival among women with high-risk early breast cancer: a randomized clinical trial. JAMA 316:1888–1896
Gogas H, Dafni U, Karina M, Papadimitriou C, Batistatou A, Bobos M et al (2012) Postoperative dose dense sequential versus concomitant administration of epirubicin and paclitaxel in patients with node-positive breast cancer: 5-year results of the Hellenic Cooperative Oncology Group HE 10/00 phase III Trial. Breast Cancer Res Treat 132:609–619
Swain SM, Tang G, Geyer CE Jr, Rastogi P, Atkins JN, Donnellan PP et al (2013) Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: the NSABP B-38 trial. J Clin Oncol 31:3197–3204
Therasse P, Mauriac L, Welnicka-Jaskiewicz M, Bruning P, Cufer T, Bonnefoi H et al (2003) Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with dose intensified epirubicin and cyclophosphamide plus filgrastim as neoadjuvant treatment in locally advanced breast cancer. An EORTC-NCIC-SAKK multicenter study. J Clin Oncol 21:843–850
Linden HM, Haskell CM, Green SJ, Osborne CK, Sledge GW Jr, Shapiro CL et al (2007) Sequenced compared with simultaneous anthracycline and cyclophosphamide in high-risk stage I and II breast cancer: final analysis from INT-0137 (S9313). J Clin Oncol 25:656–661
Mavroudis D, Matikas A, Malamos N, Papakotoulas P, Kakolyris S, Boukovinas I et al (2016) Dose-dense FEC followed by docetaxel versus docetaxel plus cyclophosphamide as adjuvant chemotherapy in women with HER2-negative, axillary lymph node-positive early breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol 27:1873–1878
Bonilla L, Ben-Aharon I, Vidal L, Gafter-Gvili A, Leibovici L, Stemmer SM (2010) Dose-dense chemotherapy in nonmetastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. J Natl Cancer Inst 102:1845–1854
Amir E, Ocana A, Freedman O, Clemons M, Seruga B (2010) Chemotherapy: dose-dense treatment for triple-negative breast cancer. Nat Rev Clin Oncol 7:79–80
Von Minckwitz G, Raab G, Caputo A, Schütte M, Hilfrich J, Blohmer JU et al (2005) Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol 23:2676–2685
Kümmel S, Krocker J, Kohls A, Breitbach GP, Morack G, Budner M et al (2006) Randomised trial: survival benefit and safety of adjuvant dose-dense chemotherapy for node-positive breast cancer. Br J Cancer 94:1237–1244
Del Mastro L, De Placido S, Bruzzi P, De Laurentiis M, Boni C, Cavazzini G et al (2015) Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2 × 2 factorial, randomised phase 3 trial. Lancet 385:1863–1872
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Gray R, Bradley R, Braybrooke J, Davies C, Pan H, Peto R, et al. (2017) Increasing the dose density of adjuvant chemotherapy by shortening intervals between courses or by sequential drug administration significantly reduces both disease recurrence and breast cancer mortality: an EBCTCG meta-analysis of 21,000 women in 16 randomised trials, Presented at San Antonio Breast Cancer Symposium 2017, GS1-01
Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17:2815–2834
Deeks JJ (2001) Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ 323:157–162
Stanley TD, Doucouliagos H (2017) Neither fixed nor random: weighted least squares meta-regression. Res Synth Methods 8:19–42
Burnell M, Levine MN, Chapman JA, Bramwell V, Gelmon K, Walley B et al (2010) Cyclophosphamide, epirubicin, and Fluorouracil versus dose-dense epirubicin and cyclophosphamide followed by paclitaxel versus doxorubicin and cyclophosphamide followed by paclitaxel in node-positive or high-risk node-negative breast cancer. J Clin Oncol 28:77–82
Moebus V, Jackisch C, Lueck HJ, du Bois A, Thomssen C, Kurbacher C et al (2010) Intense dose-dense sequential chemotherapy with epirubicin, paclitaxel, and cyclophosphamide compared with conventionally scheduled chemotherapy in high-risk primary breast cancer: mature results of an AGO phase III study. J Clin Oncol 28:2874–2880
Untch M, Möbus V, Kuhn W, Muck BR, Thomssen C, Bauerfeind I et al (2009) Intensive dose-dense compared with conventionally scheduled preoperative chemotherapy for high-risk primary breast cancer. J Clin Oncol 27:2938–2945
Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ et al (2006) Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 295:1658–1667
Petrelli F, Cabiddu M, Coinu A, Borgonovo K, Ghilardi M, Lonati V et al (2015) Adjuvant dose-dense chemotherapy in breast cancer: a systematic review and meta-analysis of randomized trials. Breast Cancer Res Treat 151:251–259
NRG-BR003-A randomized phase III trial of adjuvant therapy comparing doxorubicin plus cyclophosphamide followed by weekly paclitaxel with or without carboplatin for node-positive or high-risk node-negative triple-negative invasive breast cancer. https://clinicaltrials.gov/ct2/show/NCT02488967. Accessed 1 Feb 2018
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This meta-analysis complies with the current laws.
Appendices
Appendix 1
Search algorithm
(dose-dense chemotherapy OR dense OR acceler* OR weekly OR bi-weekly OR biweekly) AND (Breast neoplasm MeSH OR ((breast OR mammary OR mamario OR seno) AND (carcinoma OR malignan* OR neoplasm OR tumor)))) AND (randomized controlled trial [pt]OR controlled clinical trial [pt]OR randomized controlled trial [mh]OR double-blind method [mh] OR single-blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR (“clinical trial”) [tw] OR singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw] AND (mask* [tw] OR blind* [tw]))) OR comparative study [mh] OR evaluation studies [mh] OR follow up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospective* [tw] OR volunteer* [tw] NOT (animals [mh] NOT humans [mh]).
Appendix 2
See Table 4.
Rights and permissions
About this article
Cite this article
Goldvaser, H., Majeed, H., Ribnikar, D. et al. Influence of control group therapy on the benefit from dose-dense chemotherapy in early breast cancer: a systemic review and meta-analysis. Breast Cancer Res Treat 169, 413–425 (2018). https://doi.org/10.1007/s10549-018-4710-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4710-5