Abstract
Purpose
To determine the prognostic role of tamoxifen therapy for patients with ductal carcinoma in situ (DCIS) according to molecular subtypes.
Methods
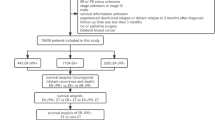
Data of 14,944 patients with DCIS were analyzed. Molecular subtypes were classified into four categories based on expression of estrogen receptor (ER)/progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2). Kaplan–Meier estimator was used for overall survival analysis while Cox proportional hazards model was used for univariate and multivariate analyses.
Results
Luminal A subtype (ER/PR+, HER2−) showed higher (P = .009) survival rate than triple-negative (TN) subtype. Tamoxifen therapy group showed superior (P < .001) survival than no-tamoxifen therapy group. It had survival benefit only for luminal A subtype (P = .001). Tamoxifen therapy resulted in higher survival rate in subgroups with positive ER (P = .006), positive PR (P = .009), and negative HER2 (P < .001). In luminal A subtype, tamoxifen therapy showed lower hazard ratio (HR) compared to no-tamoxifen therapy (HR, 0.420; 95% CI 0.250–0.705; P = .001). Tamoxifen therapy was a significant independent factor by multivariate analysis (HR, 0.538; 95% CI 0.306–0.946; P = .031) as well as univariate analysis.
Conclusion
Tamoxifen therapy group showed superior prognosis than the no-tamoxifen therapy group. Its prognostic influence was only effective for luminal A subtype. Patients with luminal A subtype showed higher survival rate than those with TN subtype. Active tamoxifen therapy is recommended for DCIS patients with luminal A subtype, and routine tests for ER, PR, and HER2 should be considered for DCIS.


Similar content being viewed by others
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DCIS:
-
Ductal carcinoma in situ
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hazard ratio
- HRc:
-
Hormone receptor
- KBCR:
-
Korean Breast Cancer Registry
- NSABP:
-
National Surgical Adjuvant Breast and Bowel Project
- PR:
-
Progesterone receptor
- EER:
-
Surveillance, epidemiology, and end results
- TN:
-
Triple negative
- UK/ANZ:
-
United Kingdom, Australia, and New Zealand
References
Worni M, Akushevich I, Greenup R et al (2015) Trends in treatment patterns and outcomes for ductal carcinoma in situ. J Natl Cancer Inst 107:djv263
Ernster VL, Ballard-Barbash R, Barlow WE et al (2002) Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst 94:1546–1554
Narod SA, Iqbal J, Giannakeas V et al (2015) Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol 1:888–896
NCCN guideline for breast cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf, Version 2.2017, Accessed 6 June, 2017
Allred DC, Anderson SJ, Paik S et al (2012) Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol 30:1268–1273
Fisher B, Dignam J, Wolmark N et al (1998) Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol 16:441–452
Fisher ER, Dignam J, Tan-Chiu E et al (1999) Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer 86:429–438
Fisher B, Land S, Mamounas E et al (2001) Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol 28:400–418
Wapnir IL, Dignam JJ, Fisher B et al (2011) Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst 103:478–488
Houghton J, George WD, Cuzick J et al (2003) Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet 362:95–102
Cuzick J, Sestak I, Pinder SE et al (2011) Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol 12:21–29
Ahn SH, Son BH, Kim SW et al (2007) Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea–a report from the Korean Breast Cancer Society. J Clin Oncol 25:2360–2368
Moon HG, Han W, Noh DY (2009) Underweight and breast cancer recurrence and death: a report from the Korean Breast Cancer Society. J Clin Oncol 27:5899–5905
Moon HG, Han W, Noh DY (2010) Comparable survival between pN0 breast cancer patients undergoing sentinel node biopsy and extensive axillary dissection: a report from the Korean Breast Cancer Society. J Clin Oncol 28:1692–1699
Park EH, Min SY, Kim Z et al (2017) Basic facts of breast cancer in Korea in 2014: the 10-year overall survival progress. J Breast Cancer 20:1–11
Hammond ME, Hayes DF, Dowsett M et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795
Wolff AC, Hammond ME, Hicks DG et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Wu Q, Li J, Sun S et al (2017) Breast carcinoma in situ: an observational study of tumor subtype, treatment and outcomes. Oncotarget 8:2361–2371
Yu KD, Wu LM, Liu GY et al (2011) Different distribution of breast cancer subtypes in breast ductal carcinoma in situ (DCIS), DCIS with microinvasion, and DCIS with invasion component. Ann Surg Oncol 18:1342–1348
Nguyen PL, Taghian AG, Katz MS et al (2008) Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol 26:2373–2378
Lowery AJ, Kell MR, Glynn RW et al (2012) Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat 133:831–841
Staley H, McCallum I, Bruce J (2014) Postoperative Tamoxifen for ductal carcinoma in situ: Cochrane systematic review and meta-analysis. Breast 23:546–551
Acknowledgements
This article was supported by the Korean Breast Cancer Society (WA35-20170205-01).
Grant sponsor
The research for this manuscript was not financially supported and none of the authors had any relevant financial relationships.
We appreciate valuable discussion from the members of the Boramae hospital Breast cancer Study group (BBS).
Ki-Tae Hwang, MD, PhD1, Bo Kyung Koo, MD, PhD2, Young A Kim, MD, PhD3, Jongjin Kim, MD1, Eun Youn Roh, MD, PhD4, Sung Bae Park, MD5, Jin Hyun Park, MD, MS2, Han Mo Sung, RN1, Bumjo Oh, MD6, So Won Oh, MD, PhD7, Sohee Oh, PhD8, Jong Yoon Lee, MD, MS9, Ji Hyun Chang, MD, PhD10, Se Hee Jung, MD, PhD11, Young Jun Chai, MD, MS1, In Sil Choi, MD, PhD2, A Jung Chu, MD9, Kyu Ri Hwang, MD, PhD12, Eunjoo Hwang1.
1Department of Surgery; 2Department of Internal Medicine; 3Department of Pathology; 4Department of Laboratory Medicine; 5Department of Neurosurgery; 6Department of Family Medicine; 7Department of Nuclear Medicine; 7Department of Biostatistics; 7Department of Radiology; 10Department of Radiation Oncology; 11Department of Rehabilitation Medicine; 12Department of Obstetrics and Gynecology.
All BBS members are from Seoul National University Boramae Medical Center (39, Boramae-Gil, Dongjak-gu, Seoul, 156-707, Republic of Korea).
List of Korean Breast Cancer Society members who contributed to this study:
Sei Hyun Ahn1, Dong-Young Noh2, Seok Jin Nam3, Eun Sook Lee4, Byeong-Woo Park5, Woo Chul Noh6, Jung Han Yoon7, Soo Jung Lee8, Eun Kyu Lee9, Joon Jeong10, Sehwan Han11, Ho Yong Park12, Nam-Sun Paik13, Young Tae Bae14, Hyouk Jin Lee15, Heung kyu Park16, Seung Sang Ko17, Byung Joo Song18, Young Jin Suh19, Sung Hoo Jung20, Se Heon Cho21, Sei Joong Kim22, Se Jeong Oh23, Byung Kyun Ko24, Ku Sang Kim25, Chanheun Park26, Jong-Min Baek27, Ki-Tae Hwang28, Il-Sung Chang29, Jeoung Won Bae30, Jeong-Soo Kim31, Sun Hee Kang32, Geumhee Gwak33, Jee Hyun Lee34, Tae Hyun Kim35, Myungchul Chang36, Sung Yong Kim37, Jung Sun Lee38, Jeong-Yoon Song39, Hai Lin Park40, Sun Young Min41, Jung-Hyun Yang42, Sung Hwan Park43, Woo-Chan Park44, Lee Su Kim45, Dong Won Ryu46, Kweon Cheon Kim47, Min Sung Chung48, Hee Boong Park49, Cheol Wan Lim50, Un Jong Choi51, Beom Seok Kwak 52, Young Sam Park53, Hyuk Jai Shin54, Young Jin Choi55, Doyil Kim56, Airi Han57, Jong Hyun Koh58, Sangyong Choi59, Daesung Yoon60, Soo Youn Choi61, Shin Hee Chul62, Jae Il Kim63, Jae Hyuck Choi64, Jin Woo Ryu65, Chang Dae Ko66, Il Kyun Lee67, Dong Seok Lee68, Seunghye Choi69, Youn Ki Min70, Young San Jeon71, Eun-Hwa Park72
1Asan Medical Center; 2Seoul National University Hospital; 3Soonchunhyang University College of Medicine Cheonan Hospital; 4National Cancer Center; 5Yonsei University Severance Hospital; 6Korea Cancer Center Hospital; 7Chonnam National University Hwasun Hospital; 8Yeungnam University Medical Center; 9Seoul National University Bundang Hospital; 10Yonsei University Gangnam Severance Hospital; 11Ajou University School of Medicine; 12Kyungpook National University Medical Center; 13Ewha Womans University Mokdong Hospital; 14Pusan National University Hospital; 15Saegyaero Hospital; 16Gachon University Gil Hospital; 17Dankook University Cheil General Hospital and Women’s Healthcare Center; 18The Catholic University of Korea Seoul St. Mary’s Hospital; 19The Catholic University of Korea St. Vincent’s Hospital; 20Chonbuk National University Hospital; 21Dong-A University Hospital; 22Inha University Hospital; 23The Catholic University of Korea Incheon St. Mary’s Hospital; 24Ulsan University Hospital; 25Ulsan City Hospital; 26Sungkyunkwan University Kangbuk Samsung Hospital; 27The Catholic University of Korea Bucheon St. Mary’s Hospital; 28Seoul National University Boramae Medical Center; 29Chungnam National University Hospital; 30Korea University Anam Hospital; 31The Catholic University of Korea Uijeongbu St. Mary’s Hospital; 32Keimyung University School of Medicine Dongsan Medical Center; 33Inje University Sanggye Paik Hospital; 34Soonchunhyang University Seoul Hospital; 35Inje University Busan Paik Hospital; 36Dankook University Hospital; 37Soonchunhyang University College of Medicine Cheonan Hospital; 38Inje University College of Medicine Haeundae Paik Hospital; 39Kyung Hee University Hospital at Gangdong; 40Gangnam CHA University Hospital; 41Kyung Hee University Medical Center; 42Konkuk University Medical Center; 43Catholic University of Daegu Hospital; 44The Catholic University of Korea Yeouido St. Mary’s Hospital; 45Hallym University Hallym Sacred Heart Hospital; 46Kosin University Gospel Hospital; 47Chosun University Hospital; 48Hanyang University Seoul Hospital; 49Park Hee Boong Surgical Clinic; 50Soonchunhyang University Bucheon Hospital; 51Wonkwang University Hospital; 52Dongguk University Ilsan Hospital; 53Presbyterian Medical Center; 54Myongji Hospital; 55Chungbuk National University Hospital; 56MizMedi Hospital; 57Yonsei University Wonju Severance Christian Hospital; 58Cheongju St. Mary’s Hospital; 59Gwangmyung Sung Ae Hospital; 60Konyang University Hospital; 61Hallym University Kangdong Sacred Heart Hospital; 62ChungAng University Hospital; 63Inje University Ilsan Paik Hospital; 64Jeju National University Hospital; 65Chungmu Genral Hospital; 66Dr. Ko’s breast Clinic, 67The Catholic Kwandong University International St. Mary’s Hospital; 68Bun Hong Hospital; 69The Catholic University of Korea St. Paul’s Hospital; 70Cheju Halla General Hospital; 71Goo Hospital; 72Ulsan University Gangneung Asan Hospital.
Funding
The research for this manuscript was not financially supported and none of the authors had any relevant financial relationships.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that no actual or potential conflict of interest exists. The institutional review boards approved this study (Seoul National University Boramae Medical Center, 07-2017-6).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required.
Research involving Human Participants and/or Animals
This article does not contain any studies with animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hwang, KT., Kim, EK., Jung, S.H. et al. Tamoxifen therapy improves overall survival in luminal A subtype of ductal carcinoma in situ: a study based on nationwide Korean Breast Cancer Registry database. Breast Cancer Res Treat 169, 311–322 (2018). https://doi.org/10.1007/s10549-018-4681-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4681-6