Abstract
Purpose
To evaluate whether adding humanized monoclonal insulin growth factor-1 receptor (IGF-1R) antibody (dalotuzumab) to mammalian target of rapamycin (mTOR) inhibitor (ridaforolimus) plus aromatase inhibitor (exemestane) improves outcomes in patients with estrogen receptor (ER)-positive advanced/metastatic breast cancer.
Methods
This randomized, open-label, phase II trial enrolled 80 postmenopausal women with high-proliferation (Ki67 index staining ≥15%), ER-positive breast cancer that progressed after a non-steroidal aromatase inhibitor (NCT01605396). Randomly assigned patients were given oral ridaforolimus 10 mg QD 5 ×/week, intravenous dalotuzumab 10 mg/kg/week, and oral exemestane 25 mg/day (R/D/E, n = 40), or ridaforolimus 30 mg QD 5 ×/week and exemestane 25 mg/day (R/E; n = 40). Primary end point was progression-free survival (PFS).
Results
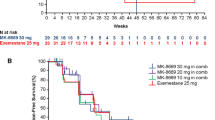
Median PFS was 23.3 weeks for R/D/E versus 31.9 weeks for R/E (hazard ratio 1.18; 80% CI 0.81–1.72; P = 0.565). Grade 3–5 adverse events were reported in 67.5% of patients in the R/E arm and 59.0% in the R/D/E arm. Stomatitis (95.0 vs. 76.9%; P = 0.021) and pneumonitis (22.5 vs. 5.1%; P = 0.027) occurred more frequently in the R/E than the R/D/E arm; hyperglycemia (27.5 vs. 28.2%) occurred at a similar rate.
Conclusions
R/D/E did not improve PFS compared with R/E. Because the PFS reported for R/E was similar to that reported for everolimus plus exemestane in patients with advanced breast cancer, it is possible that lower-dose ridaforolimus in the R/D/E arm (from overlapping toxicities with IGF1R inhibitor) contributed to lack of improved PFS.


Similar content being viewed by others
References
Johnston SR (2006) Clinical efforts to combine endocrine agents with targeted therapies against epidermal growth factor receptor/human epidermal growth factor receptor 2 and mammalian target of rapamycin in breast cancer. Clin Cancer Res 12:1061s–1068s
Burstein HJ (2011) Novel agents and future directions for refractory breast cancer. Semin Oncol 38(suppl 2):S17–S24
Quek R, Wang Q, Morgan JA et al (2011) Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res 17:871–879
Di Cosimo S, Sathyanarayanan S, Bendell JC et al (2015) Combination of the mTOR inhibitor ridaforolimus and the anti-IGF1R monoclonal antibody dalotuzumab: preclinical characterization and phase I clinical trial. Clin Cancer Res 21:49–59
Elit L (2006) Drug evaluation: aP-23573—an mTOR inhibitor for the treatment of cancer. IDrugs 9:636–644
Rivera VM, Squillace RM, Miller D et al (2011) Ridaforolimus (AP23573; MK-8669), a potent mTOR inhibitor, has broad antitumor activity and can be optimally administered using intermittent dosing regimens. Mol Cancer Ther 10:1059–1071
Sun SY, Rosenberg LM, Wang X et al (2005) Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 65:7052–7058
Baselga J, Semiglazov V, van Dam P et al (2009) Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27:2630–2637
Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, Houghton PJ (2009) The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res 69:7662–7671
Broussas M, Dupont J, Gonzalez A et al (2009) Molecular mechanisms involved in activity of h7C10, a humanized monoclonal antibody, to IGF-1 receptor. Int J Cancer 124:2281–2293
O’Reilly KE, Rojo F, She QB et al (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66:1500–1508
Baselga J, Morales SM, Awada A et al (2013) A phase 2 study of ridaforolimus (RIDA) and dalotuzumab (DALO) in estrogen receptor positive (ER+) breast cancer [Abstract]. Cancer Res 73(24):TPS110
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Ellis PM, Shepherd FA, Laurie SA et al (2014) NCIC CTG IND.190 phase I trial of dalotuzumab (MK-0646) in combination with cisplatin and etoposide in extensive-stage small-cell lung cancer. J Thorac Oncol 9:410–413
Fierz Y, Novosyadlyy R, Vijayakumar A, Yakar S, LeRoith D (2010) Mammalian target of rapamycin inhibition abrogates insulin-mediated mammary tumor progression in type 2 diabetes. Endocr Rel Cancer 17:941–951
Ray-Coquard I, Haluska P, O’Reilly S et al (2013) A multicenter open-label phase II study of the efficacy and safety of ganitumab (AMG 479), a fully human monoclonal antibody against insulin-like growth factor type 1 receptor (IGF-1R) as second-line therapy in patients with recurrent platinum-sensitive ovarian cancer. J Clin Oncol 31:5515
Ryan PD, Neven P, Dirix LY et al (2016) Safety of the anti-IGF-1R antibody CP-751,871 in combination with exemestane in patients with advanced breast cancer. Cancer Res 69(2):2136
Okusaka T, Ikeda M, Fukutomi A et al (2014) Safety, tolerability, pharmacokinetics and antitumor activity of ganitumab, an investigational fully human monoclonal antibody to insulin-like growth factor type 1 receptor, combined with gemcitabine as first-line therapy in patients with metastatic pancreatic cancer: a phase 1b study. Jpn J Clin Oncol 44:442–447
Miettinen O, Nurminen M (1985) Comparative analysis of two rates. Stat Med 4:213–226
Haluska P, Shaw HM, Batzel GN et al (2007) Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res 13:5834–5840
Yee D, Paoloni M, Van’t Veer L et al (2016) The evaluation of ganitumab/metformin plus standard neoadjuvant therapy in high-risk breast cancer: results from the I-SPY 2 trial. Presented at: San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract P6-11-04
Gradishar WJ, Yardley DA, Layman R et al (2016) Clinical and translational results of a phase II, randomized trial of an anti-IGF-1R (cixutumumab) in women with breast cancer that progressed on endocrine therapy. Clin Cancer Res 22:301–309
Yardley DA, Noguchi S, Pritchard KI et al (2013) Everolimus plus exemestane in postmenopausal patients with HR breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther 30:870–884
National Comprehensive Cancer Network, Inc (2014) NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. version 1. 2014. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 22 Nov 2016
Boers-Doets CB, Raber-Durlacher JE, Treister NS et al (2013) Mammalian target of rapamycin inhibitor-associated stomatitis. Future Oncol 9:1883–1892
Martins F, de Oliveira MA, Wang Q et al (2013) A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. Oral Oncol 49:293–298
Pilotte AP, Hohos MB, Polson KM, Huftale TMn, Treister N (2011) Managing stomatitis in patients treated with Mammalian target of rapamycin inhibitors. Clin J Oncol Nurs 15:E83–E89
Baselga J, Campone M, Piccart M et al (2012) Everolimus in postmenopausal hormone receptor-positive advanced breast cancer. N Engl J Med 366:520–529
Acknowledgements
We thank the patients and their families for their participation and support during the study as well as the study center staff and principal investigators. Editorial support was provided by Tim Ibbotson, PhD, of ApotheCom and was funded by Merck & Co., Inc., Kenilworth, NJ.
Funding
This work was supported by a grant from Merck & Co., Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
JC has received fees for lectures and consulting from Roche, Celgene, Novartis, and Eisai. HSR has received research support from Merck & Co., Inc. and Novartis. ART received grants from Merck & Co., Inc. for conducting this study. AD, CKG, EI, MBJ, DJM, and ZW are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ and may hold stock or stock options in the company. MF has received grants from Merck Sharp & Dohme Corp. and AstraZeneca, grants and non-financial support from Novartis, and F. Hoffmann-La Roche, and non-financial support from Merck Sharp & Dohme Corp. NA, JB, JLB, MC, LE, AM, JR, SMM, OT, and KHP have nothing to disclose.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Independent ethics committees reviewed and approved the protocol and applicable amendments for each institution.
Informed consent
Written informed consent was obtained from all participants.
Rights and permissions
About this article
Cite this article
Rugo, H.S., Trédan, O., Ro, J. et al. A randomized phase II trial of ridaforolimus, dalotuzumab, and exemestane compared with ridaforolimus and exemestane in patients with advanced breast cancer. Breast Cancer Res Treat 165, 601–609 (2017). https://doi.org/10.1007/s10549-017-4375-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4375-5