Abstract
Purpose
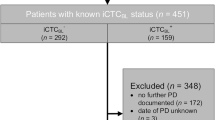
While intact circulating tumor cells (iCTC) have independent negative prognostic impact on patients with metastatic breast cancer (MBC), the prognostic relevance of apoptotic CTC (aCTC) has not been validated in larger patient cohorts. This study assessed aCTC and iCTC statuses at baseline (CTCBL) and CTC kinetics (CTCKIN) as changes from CTCBL to one completed treatment cycle for their utility in predicting response, progression-free survival (PFS), and overall survival (OS) in MBC.
Methods
Status of iCTC and aCTC was prospectively assessed in 442 patients using the CellSearch™ system. Different cutoffs were analyzed both for iCTC and aCTC (≥5, ≥10, ≥25 and ≥50 CTC/7.5 ml). CTCKIN were characterized by ≥25 % changes in CTC counts.
Results
Numbers of iCTC and aCTC at baseline correlated strongly (r = 0.7). For iCTCBL positive patients, additional detection of aCTCBL had a significant prognostic impact on OS (aCTCBL positive 10.3 vs. aCTCBL negative 16.4 months, p = 0.012). Worst prognosis for OS was observed in patients with ≥50 iCTC/7.5 ml and simultaneously detected aCTC. Determination of aCTCKIN showed stronger discriminating power than iCTCKIN, with higher PFS and OS for the group with decreasing CTCs (PFS 7.7 vs. 6.1; OS 22.2 vs. 16.4).
Conclusions
Intact and aCTC are predictive of outcome in MBC. Apoptotic CTC counts ≥ 5/7.5 ml in conjunction with iCTC at baseline have an independent unfavorable prognostic impact on OS. Decreasing aCTCKIN at ≥ 5/7.5 ml in serial enumeration is associated with favorable outcome. Therefore, separate enumeration of iCTC and aCTC is useful in tailoring systemic treatment.








Similar content being viewed by others
Abbreviations
- 1C:
-
One cycle of systemic chemotherapy
- aCTC:
-
Apoptotic circulating tumor cell(s)
- BL:
-
Baseline
- CHT:
-
Chemotherapy
- CI:
-
Confidence interval
- CTC:
-
Circulating tumor cell(s)
- DTC:
-
Disseminated tumor cell(s)
- EMT:
-
Epithelial–mesenchymal transition
- EpCAM:
-
Epithelial cell adhesion molecule
- iCTC:
-
Intact circulating tumor cell(s)
- KIN:
-
Kinetics
- MBC:
-
Metastatic breast cancer
- NA:
-
Not available/not applicable
- NCT:
-
National Center for Tumor Diseases, Heidelberg, Germany
- NST:
-
Neoadjuvant systemic therapy
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- STD:
-
Standard deviation
References
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW et al (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8):781–791
Giordano A, Gao H, Anfossi S, Cohen E, Mego M, Lee BN, Tin S, De Laurentiis M, Parker CA, Alvarez RH et al (2012) Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol Cancer Ther 11(11):2526–2534
Wallwiener M, Riethdorf S, Hartkopf AD, Modugno C, Nees J, Madhavan D, Sprick MR, Schott S, Domschke C, Baccelli I et al (2014) Serial enumeration of circulating tumor cells predicts treatment response and prognosis in metastatic breast cancer: a prospective study in 393 patients. BMC Cancer 14:512
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12(14 Pt 1):4218–4224
Giuliano M, Giordano A, Jackson S, Hess KR, De Giorgi U, Mego M, Handy BC, Ueno NT, Alvarez RH, De Laurentiis M et al (2011) Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast Cancer Res 13(3):R67
Dawood S, Broglio K, Valero V, Reuben J, Handy B, Islam R, Jackson S, Hortobagyi GN, Fritsche H, Cristofanilli M (2008) Circulating tumor cells in metastatic breast cancer: from prognostic stratification to modification of the staging system? Cancer 113(9):2422–2430
Fehm T, Sagalowsky A, Clifford E, Beitsch P, Saboorian H, Euhus D, Meng SD, Morrison L, Tucker T, Lane N et al (2002) Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin Cancer Res 8(7):2073–2084
Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bauerle T, Wallwiener M et al (2013) Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 31(6):539–544
Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen LW et al (2006) Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res 12(21):6403–6409
Wallwiener M, Hartkopf AD, Baccelli I, Riethdorf S, Schott S, Pantel K, Marme F, Sohn C, Trumpp A, Rack B et al (2013) The prognostic impact of circulating tumor cells in subtypes of metastatic breast cancer. Breast Cancer Res Treat 137(2):503–510
Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, Tejwani S, Schott AF, O’Rourke MA, Lew DL et al (2014) Circulating tumor cells and response to chemotherapy in metastatic breast cancer: sWOG S0500. J Clin Oncol 32(31):3483–3489
Hartkopf AD, Wagner P, Wallwiener D, Fehm T, Rothmund R (2011) Changing levels of circulating tumor cells in monitoring chemotherapy response in patients with metastatic breast cancer. Anticancer Res 31(3):979–984
Fehm T, Hoffmann O, Aktas B, Becker S, Solomayer EF, Wallwiener D, Kimmig R, Kasimir-Bauer S (2009) Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res 11(4):R59
Wallwiener M, Hartkopf AD, Riethdorf S, Nees J, Sprick MR, Schonfisch B, Taran FA, Heil J, Sohn C, Pantel K et al (2015) The impact of HER2 phenotype of circulating tumor cells in metastatic breast cancer: a retrospective study in 107 patients. BMC Cancer 15:403
Mehes G, Witt A, Kubista E, Ambros PF (2001) Circulating breast cancer cells are frequently apoptotic. Am J Pathol 159(1):17–20
Rossi E, Basso U, Celadin R, Zilio F, Pucciarelli S, Aieta M, Barile C, Sava T, Bonciarelli G, Tumolo S et al (2010) M30 neoepitope expression in epithelial cancer: quantification of apoptosis in circulating tumor cells by Cell Search analysis. Clin Cancer Res 16(21):5233–5243
Kallergi G, Konstantinidis G, Markomanolaki H, Papadaki MA, Mavroudis D, Stournaras C, Georgoulias V, Agelaki S (2013) Apoptotic circulating tumor cells in early and metastatic breast cancer patients. Mol Cancer Ther 12(9):1886–1895
Krawczyk N, Hartkopf A, Banys M, Meier-Stiegen F, Staebler A, Wallwiener M, Rohm C, Hoffmann J, Hahn M, Fehm T (2014) Prognostic relevance of induced and spontaneous apoptosis of disseminated tumor cells in primary breast cancer patients. BMC Cancer 14:394
Russo A, Terrasi M, Agnese V, Santini D, Bazan V (2006) Apoptosis: a relevant tool for anticancer therapy. Ann Oncol 17(Suppl 7 vii):115–123
Smerage JB, Budd GT, Doyle GV, Brown M, Paoletti C, Muniz M, Miller MC, Repollet MI, Chianese DA, Connelly MC et al (2013) Monitoring apoptosis and Bcl-2 on circulating tumor cells in patients with metastatic breast cancer. Mol Oncol 7(3):680–692
Fehm T, Becker S, Becker-Pergola G, Sotlar K, Gebauer G, Durr-Storzer S, Neubauer H, Wallwiener D, Solomayer EF (2006) Presence of apoptotic and nonapoptotic disseminated tumor cells reflects the response to neoadjuvant systemic therapy in breast cancer. Breast Cancer Res 8(5):R60
Hartkopf AD, Taran FA, Wallwiener M, Hagenbeck C, Melcher C, Krawczyk N, Hahn M, Wallwiener D, Fehm T (2013) The presence and prognostic impact of apoptotic and nonapoptotic disseminated tumor cells in the bone marrow of primary breast cancer patients after neoadjuvant chemotherapy. Breast Cancer Res 15(5):R94
Riethdorf S, Muller V, Zhang L, Rau T, Loibl S, Komor M, Roller M, Huober J, Fehm T, Schrader I et al (2010) Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res 16(9):2634–2645
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Riethdorf S, Pantel K (2008) Disseminated tumor cells in bone marrow and circulating tumor cells in blood of breast cancer patients: current state of detection and characterization. Pathobiology 75(2):140–148
Cristofanilli M (2009) The biological information obtainable from circulating tumor cells. Breast 18(Suppl 3):S38–S40
R: A Language and environment for statistical computing. http://www.r-project.org
Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D et al (2004) Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 10(24):8152–8162
Fehm T, Muller V, Aktas B, Janni W, Schneeweiss A, Stickeler E, Lattrich C, Lohberg CR, Solomayer E, Rack B et al (2010) HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat 124(2):403–412
Acknowledgments
The authors gratefully acknowledge all patients whose data were used in this study. We also thank the medical and nursing staff, especially Martina Scharpff, at the National Center for Tumor Diseases (NCT; Heidelberg) for excellent management and care of our patients; the NCT laboratory staff; and Antje Andreas; Cornelia Coith; and Oliver Mauermann (Hamburg), who provided excellent technical assistance with the CTC determinations. This study was supported by NCT in-house funds, made available to AS and AT, and by grants to AT from the BioRN Leading Edge Cluster “Molecular and Cell Based Medicine” (BRN 02GS1893), supported by the German Federal Ministry of Education and Research (BMBF), Berlin, Germany (BMBF N02/74829), and the Dietmar Hopp Foundation. Moreover, this study was supported by the ERC-2010-AdG_20100317 DISSECT to KP. We acknowledge the financial support from the German Research Foundation (DFG) and Ruprecht-Karls-Universität Heidelberg through the funding program for Open Access Publishing.
Authors’ contributions
MW, ADH, SR, KP, AT, and AS jointly conceived the study and developed its design. MW and AS supervised the study. SR and KP developed the methodology. TMD, SR, JN, ADH, MRS, BS, CS, KP, AT, AS, and MW participated in patient recruitment, patient management, clinical data collection, sample collection, and sample analysis. BS and TMD organized and reported the data, constructed the databases, and conducted data management. BS performed the statistical analysis. TMD, JN, ADH, SS, SYB, SR, FS, CD, MRS, BS, CS, AT, AS, and MW participated in data analysis and interpretation. TMD, JN, ADH, BS, AS, and MW drafted the manuscript. MW, SR, MRS, CS, KP, SR, SS, TMD, ADH, BS, SYB, AS, and AT revised the draft manuscript for important intellectual input. MW prepared the final manuscript. All the authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Thomas M. Deutsch and Sabine Riethdorf are joint first authors. Andreas Schneeweiss and Markus Wallwiener are joint senior authors.
Thomas M. Deutsch, Sabine Riethdorf, Andreas Schneeweiss, and Markus Wallwiener have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (EPS 404 kb)
Supplementary Fig. 1 Kaplan–Meier plots for OS with different cutoff values for iCTCKIN and aCTCKIN. OS by CTC status between baseline and first cycle in 252 patients with MBC: <5 CTC (<5 CTC in cells in both CTCBL and CTC1C), stable (<25 % change), decrease (≥25 % decrease), and increase (≥25 % increase)
Supplementary material 2 (EPS 342 kb)
Supplementary Fig. 2 Kaplan–Meier plots for PFS with different cutoff values for iCTCKIN and aCTCKIN. PFS by CTC status between baseline and first cycle in 252 patients with MBC: <5 CTC (<5 CTC in cells in both CTCBL and CTC1C), stable (<25 % change), decrease (≥25 % decrease), and increase (≥25 % increase)
Supplementary material 3 (EPS 106 kb)
Supplementary Fig. 3. Kaplan–Meier plots—Kinetic of apoptotic CTC between first cycle and progression disease. OS by CTC status between first cycle and progression disease in 189 patients with MBC: < 5 CTC (<5 CTC in cells in both CTCBL and CTC1C), stable (<25 % change), decrease (≥25 % decrease), increase (≥25 % increase)
Rights and permissions
About this article
Cite this article
Deutsch, T.M., Riethdorf, S., Nees, J. et al. Impact of apoptotic circulating tumor cells (aCTC) in metastatic breast cancer. Breast Cancer Res Treat 160, 277–290 (2016). https://doi.org/10.1007/s10549-016-3997-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3997-3