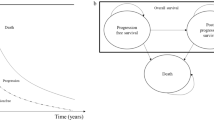
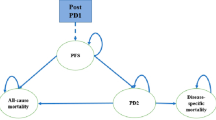
Abstract
Endocrine therapy continues to be the optimal systemic treatment for metastatic ER+HER2− breast cancer. The CDK4/6 inhibitor palbociclib combined with letrozole has recently been shown to significantly improve progression-free survival. Here we examined the cost-effectiveness of this regimen for the Swiss healthcare system. A Markov cohort simulation based on the PALOMA-1 trial (Finn et al. in Lancet Oncol 16:25–35, 2015) was used as the clinical course. Input parameters were based on summary trial data. Costs were assessed from the Swiss healthcare system perspective. Adding palbociclib to letrozole (PALLET) compared to letrozole monotherapy was estimated to cost an additional CHF342,440 and gain 1.14 quality-adjusted life years, resulting in an incremental cost-effectiveness ratio (ICER) of CHF301,227/QALY gained. In univariate sensitivity analyses, no tested variation in key parameters resulted in an ICER below a willingness-to-pay threshold of CHF100,000/QALY. PALLET had a 0 % probability of being cost-effective in probabilistic sensitivity analyses. Lowering PALLET’s price by 75 % resulted in an ICER of CHF73,995/QALY and a 73 % probability of being cost-effective. At current prices, PALLET would cost the Swiss healthcare system an additional CHF155 million/year. Palbociclib plus letrozole cannot be considered cost-effective for the first-line treatment of patients with metastatic breast cancer in the Swiss healthcare system.

Similar content being viewed by others
References
Chen L, Linden HM, Anderson BO, Li CI (2014) Trends in 5-year survival rates among breast cancer patients by hormone receptor status and stage. Breast Cancer Res Treat 147(3):609–616. doi:10.1007/s10549-014-3112-6
Mendes D, Alves C, Afonso N, Cardoso F, Passos-Coelho JL, Costa L, Andrade S, Batel-Marques F (2015) The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer—a systematic review. Breast Cancer Res 17:140. doi:10.1186/s13058-015-0648-2
Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, Barrios CH, Bergh J, Biganzoli L, Blackwell KL, Cardoso MJ, Cufer T, El Saghir N, Fallowfield L, Fenech D, Francis P, Gelmon K, Giordano SH, Gligorov J, Goldhirsch A, Harbeck N, Houssami N, Hudis C, Kaufman B, Krop I, Kyriakides S, Lin UN, Mayer M, Merjaver SD, Nordstrom EB, Pagani O, Partridge A, Penault-Llorca F, Piccart MJ, Rugo H, Sledge G, Thomssen C, Van’t Veer L, Vorobiof D, Vrieling C, West N, Xu B, Winer E (2014) ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) dagger. Ann Oncol 25(10):1871–1888. doi:10.1093/annonc/mdu385
Partridge AH, Rumble RB, Carey LA, Come SE, Davidson NE, Di Leo A, Gralow J, Hortobagyi GN, Moy B, Yee D, Brundage SB, Danso MA, Wilcox M, Smith IE (2014) Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 32(29):3307–3329. doi:10.1200/JCO.2014.56.7479
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, Shparyk Y, Thummala AR, Voytko NL, Fowst C, Huang X, Kim ST, Randolph S, Slamon DJ (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35. doi:10.1016/S1470-2045(14)71159-3
Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, Zirkelbach JF, Yu J, Liu Q, Zhao L, Crich J, Chen XH, Hughes M, Bloomquist E, Tang S, Sridhara R, Kluetz PG, Kim G, Ibrahim A, Pazdur R, Cortazar P (2015) FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res 21(21):4760–4766. doi:10.1158/1078-0432.CCR-15-1185
Delea TE, Hawkes C, Amonkar MM, Lykopoulos K, Johnston SR (2013) Cost-effectiveness of lapatinib plus letrozole in post-menopausal women with hormone receptor-and HER2-positive metastatic breast cancer. Breast Care 8(6):429–437. doi:10.1159/000357316
Xie J, Hao Y, Zhou ZY, Qi CZ, De G, Gluck S (2015) Economic evaluations of everolimus versus other hormonal therapies in the treatment of HR(+)/HER2(−) advanced breast cancer from a US payer perspective. Clin Breast Cancer 15(5):e263–276. doi:10.1016/j.clbc.2015.04.001
Brandle M, Goodall G, Erny-Albrecht KM, Erdmann E, Valentine WJ (2009) Cost-effectiveness of pioglitazone in patients with type 2 diabetes and a history of macrovascular disease in a Swiss setting. Swiss Med Wkly 139(11–12):173–184
Diebold J, Aebi S (2014) Prognostische und prädiktive Marker beim Mammakarzinom. SZO 1:10–14
Tengs TO (2004) Cost-effectiveness versus cost-utility analysis of interventions for cancer: does adjusting for health-related quality of life really matter? Value Health 7(1):70–78
Ubel PA, Hirth RA, Chernew ME, Fendrick AM (2003) What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med 163(14):1637–1641
Raftery J (2006) Review of NICE’s recommendations, 1999–2005. BMJ 332(7552):1266–1268. doi:10.1136/bmj.332.7552.1266
Schwenkglenks M, Lippuner K (2007) Simulation-based cost-utility analysis of population screening-based alendronate use in Switzerland. Osteoporos Int 18(11):1481–1491. doi:10.1007/s00198-007-0390-4
Dedes KJ, Matter-Walstra K, Schwenkglenks M, Pestalozzi BC, Fink D, Brauchli P, Szucs TD (2009) Bevacizumab in combination with paclitaxel for HER-2 negative metastatic breast cancer: an economic evaluation. Eur J Cancer 45:1397–1406. doi:10.1016/j.ejca.2008.12.01610.1016/j.ejca.2008.12.016
Joerger M, Matter-Walstra K, Fruh M, Kuhnel U, Szucs T, Pestalozzi B, Schwenkglenks M (2011) Addition of cetuximab to first-line chemotherapy in patients with advanced non-small-cell lung cancer: a cost-utility analysis. Ann Oncol 22(3):567–574. doi:10.1093/annonc/mdq431
Matter-Walstra K, Braun R, Kolb C, Ademi Z, Dummer R, Pestalozzi BC, Schwenkglenks M (2015) A cost-effectiveness analysis of trametinib plus dabrafenib as first-line therapy for metastatic BRAF V600-positive melanoma in the Swiss setting. Br J Dermatol. doi:10.1111/bjd.14152
Matter-Walstra K, Joerger M, Kuhnel U, Szucs T, Pestalozzi B, Schwenkglenks M (2012) Cost-effectiveness of maintenance pemetrexed in patients with advanced nonsquamous-cell lung cancer from the perspective of the Swiss health care system. Value Health 15(1):65–71. doi:10.1016/j.jval.2011.08.1737
Matter-Walstra KW, Dedes KJ, Schwenkglenks M, Brauchli P, Szucs TD, Pestalozzi BC (2010) Trastuzumab beyond progression: a cost-utility analysis. Ann Oncol 21(11):2161–2168. doi:10.1093/annonc/mdq250
Delea TE, Amdahl J, Chit A, Amonkar MM (2013) Cost-effectiveness of lapatinib plus letrozole in her2-positive, hormone receptor-positive metastatic breast cancer in Canada. Curr Oncol 20(5):e371–387. doi:10.3747/co.20.1394
Sebaratnam DF, Anforth R, Fernandez-Penas P (2015) Treatment specific utility-weightings are needed for cost-utility analysis in metastatic melanoma. Br J Dermatol. doi:10.1111/bjd.14264
Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG (2000) Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Mak 20(3):332–342
Durkee BY, Qian Y, Pollom EL, King MT, Dudley SA, Shaffer JL, Chang DT, Gibbs IC, Goldhaber-Fiebert JD, Horst KC (2015) Cost-effectiveness of pertuzumab in human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. doi:10.1200/JCO.2015.62.9105
Lichtenberg FR (2015) The impact of pharmaceutical innovation on premature cancer mortality in Switzerland, 1995–2012. Eur J Health Econ. doi:10.1007/s10198-015-0725-6
Lakdawalla DN, Chou JW, Linthicum MT, MacEwan JP, Zhang J, Goldman DP (2015) Evaluating expected costs and benefits of granting access to new treatments on the basis of progression-free survival in non-small-cell lung cancer. JAMA Oncol 1(2):196–202. doi:10.1001/jamaoncol.2015.0203
Acknowledgments
We thank the SAKK Coordinating Centre and Nextgenediting for reviewing our manuscript.
Funding
The Swiss State Secretariat for Education, Research and Innovation (SERI) supported this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared no potential conflicts of interest related to palbociclib or other drugs mentioned in this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matter-Walstra, K., Ruhstaller, T., Klingbiel, D. et al. Palbociclib as a first-line treatment in oestrogen receptor-positive, HER2-negative, advanced breast cancer not cost-effective with current pricing: a health economic analysis of the Swiss Group for Clinical Cancer Research (SAKK). Breast Cancer Res Treat 158, 51–57 (2016). https://doi.org/10.1007/s10549-016-3822-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3822-z