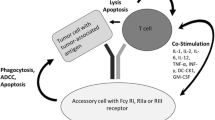
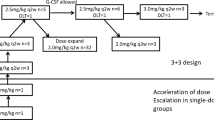
Abstract
The objectives of this phase I/II study (NCT00140738) were to evaluate the safety and clinical activity of a cancer immunotherapeutic agent (recombinant HER2 protein (dHER2) and the immunostimulant AS15) in patients with HER2-overexpressing metastatic breast cancer (MBC). Forty HER2-positive MBC patients received up to 18 doses (12q2w, 6q3w) of dHER2 immunotherapeutic, as first- or second-line therapy following response to trastuzumab-based treatment as maintenance. Toxicity was graded by the Common Terminology Criteria for Adverse Events (CTCAE) and clinical activity was evaluated by target lesion assessment according to the Response Evaluation Criteria in Solid Tumors (RECIST). Immunogenicity was assessed. The dHER2 immunotherapeutic was well tolerated: grade 1/2 adverse events (AEs) were most common. No cardiac events were observed and one patient experienced an asymptomatic decrease of left ventricular ejection fraction below the normal range (47 %). Both humoral and cellular immunogenicity to the dHER2 antigen was observed. No patient discontinued the immunizations because of AEs but 35/40 withdrew prematurely, 34 because of disease progression (24/34 before or at the tumor assessment after dose 6). One patient achieved a complete response lasting 11 months and one patient had a partial response lasting 3.5 months. Ten patients experienced stable disease ≥26 weeks with 4/10 still in stable disease at the last tumor assessment after 47 weeks. Immunization of MBC patients with the dHER2 immunotherapeutic was associated with minimal toxicity and no cardiac events. Clinical activity was observed with two objective responses and prolonged stable disease for 10/40 patients.



Similar content being viewed by others
Abbreviations
- Ab:
-
Antibody
- AE:
-
Adverse events
- BC:
-
Breast cancer
- CBR:
-
Clinical benefit rate
- CMI:
-
Cell-mediated immunogenicity
- CR:
-
Complete response
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- ECD:
-
Extracellular domain
- ECOG:
-
Eastern Cooperative Oncology Group
- ELISA:
-
Enzyme-linked immunosorbent assays
- GMC:
-
Geometric mean concentrations
- HER2:
-
Human epidermal growth factor receptor 2
- ICD:
-
Intracellular domain
- LVEF:
-
Left ventricular ejection fraction
- MBC:
-
Metastatic breast cancer
- PBMC:
-
Peripheral blood mononuclear cells
- PR:
-
Partial response
- RECIST:
-
Response evaluation criteria in solid tumors
- SAE:
-
Serious adverse event
- SD:
-
Stable disease
References
Zardavas D, Cameron D, Krop I et al (2013) Beyond trastuzumab and lapatinib: New options for HER2-positive breast cancer. Am Soc Clin Oncol Educ Book e2-e11
Goldhirsch A, Gelber RD, Piccart-Gebhart MJ et al (2013) 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label randomised controlled trial. Lancet 382:1021–1028
Andersson M, Lidbrink E, Bjerre K et al (2011) Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol 29:264–271
Higgins MJ, Baselga J (2011) Targeted therapies for breast cancer. J Clin Invest 121:3797–3803
Hamilton E, Blackwell K, Hobeika AC et al (2012) Phase I clinical trial of HER2-specific immunotherapy with concomitant HER2 kinase inhibition. J Transl Med. doi:10.1186/1479-5876-10-28
Disis ML, Pupa SM, Gralow JR et al (1997) High-titer HER2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol 15:3363–3367
Emens LA, Reilly RT, Jaffee EM (2005) Breast cancer vaccines: maximizing cancer treatment by tapping into host immunity. Endocr Relat Cancer 12:1–17
Bilusic M, Madan RA (2012) Therapeutic cancer vaccines: the latest advancement in targeted therapy. Am J Ther 19:e172–e181
Swain SM, Kim SB, Cortés J et al (2013) Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 14:461–471
Verma S, Miles D, Gianni L et al (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783–1791
Krop IE, Kim SB, Gonzalez-Martin A et al (2014) Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised open-label, phase 3 trial. Lancet Oncol 15:689–699
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Krop IE, Beeram M, Modi S et al (2010) Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol 28:2698–2704
Foy TM, Fanger GR, Hand S et al (2002) Designing HER2 vaccines. Semin Oncol 29(Suppl 11):53–61
Valabrega G, Montemurro F, Aglietta M (2007) Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol 18:977–984
Stagg J, Loi S, Divisekera U et al (2011) Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci USA 108:7142–7147
Vu T, Claret FX (2012) Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol 2:62
Shi Y, Fan X, Meng W et al (2014) Engagement of immune effector cells by trastuzumab induces HER2/ERBB2 downregulation in cancer cells through STAT1 activation. Breast Cancer Res 16:R33
Disis ML, Wallace DR, Gooley TA et al (2009) Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol 27:4685–4692
Limentani SA, Campone M, Dorval T, et al: A non-randomized dose-escalation Phase I trial of a protein-based immunotherapeutic for the treatment of breast cancer patients with HER2-overexpressing tumors. Breast Cancer Res Treat, manuscript submitted in parallel
Schlom J (2012) Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst 104:599–613
Kruit WH, Suciu S, Dreno B et al (2013) Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organization for Research and Treatment of Cancer Melanoma Group in metastatic melanoma. J Clin Oncol 31:2413–2420
Curigliano G, Perez EA (2014) Immunoscoring breast cancer: TILs remember what they target. Ann Oncol 25:1455–1456
Zitvogel L, Kepp O, Kroemer G (2011) Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 8:151–160
Sharma P, Wagner K, Wolchok JD et al (2011) Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 11:805–812
Campbell CT, Gulley JL, Oyelaran O et al (2014) Humoral response to a viral glycan correlates with survival on PROSTVAC-VF. Proc Natl Acad Sci USA 111:E1749–E1758
Garcia B, Neninger E, de la Torre A et al (2008) Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti-epidermal growth factor antibodies is related to better survival in advanced non-small-cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clin Cancer Res 14:840–846
Harrop R, Shingler W, Kelleher M et al (2010) Cross-trial analysis of immunologic and clinical data resulting from phase I and II trials of MVA-5T4 (TroVax) in colorectal, renal, and prostate cancer patients. J Immunother 33:999–1005
Harrop R, Shingler WH, McDonald M et al (2011) MVA-5T4-induced immune responses are an early marker of efficacy in renal cancer patients. Cancer Immunol Immunother 60:829–837
Miles D, Roche H, Martin M et al (2011) Phase III multicenter trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist 16:1092–1100
Ibrahim NK, Murray JL, Zhou D et al (2013) Survival advantage in patients with metastatic breast cancer receiving endocrine therapy plus sialyl Tn-KLH vaccine: Post hoc analysis of a large randomized trial. J Cancer 4:577–584
Curigliano G, Disalvatore D, Esposito A et al (2015) Risk of subsequent in situ and invasive breast cancer in human epidermal growth factor receptor 2-positive ductal carcinoma in situ. Ann Oncol 26(4):682–687
Acknowledgments
The authors thank the patients who participated in this study and their families. They also acknowledge the investigators and their clinical teams for their contribution to the study and their support and care of patients, in particular Thomas Bachelot, Henri Roche and Jaak Janssens. The authors thank the global and regional clinical operations and safety teams of GSK for their contribution to the study, the scientific writer for clinical protocol and clinical report writing and the team of statisticians at GSK for the statistical analysis. The authors thank Françoise Cormont, Valentine Wascotte and Anne-Laure Puaux for critical reading of the paper and helpful suggestions. The authors thank Niels Neymark (medical writer on behalf of GSK) for assistance in the manuscript writing, and Sophie Timmery (XPE Pharma & Science) for coordination and editorial assistance.
Author contributions
Study conception and design Vincent Brichard, Frédéric F. Lehmann, Jamila Louahed, Andrea Callegaro. Collection and assembly of data Giuseppe Curigliano, Gilles Romieu, Lionel Duck, Célia Roemer-Becuwe, Mario Roselli, Silvia Neciosup, Jean-Luc Canon. Data analysis and interpretation Vincent Brichard, Frédéric F. Lehmann, Jamila Louahed, Wivine Burny, Giuseppe Curigliano, Pedro Miguel de Sousa Alves, Andrea Callegaro, Lionel Duck, Mario Campone. Provision of study materials or patients Giuseppe Curigliano, Gilles Romieu, Silvia Neciosup, Mario Campone.
Disclosures
Employment Pedro Miguel De Sousa Alves (GSK group of companies); Frédéric F. Lehmann (GSK group of companies); Jamila Louahed (GSK group of companies); Vincent G. Brichard (GSK group of companies); Wivine Burny (GSK group of companies); Andrea Callegaro (GSK group of companies). Stock ownership Frédéric F. Lehmann (GSK group of companies); Jamila Louahed (GSK group of companies); Vincent G. Brichard (GSK group of companies); Pedro Miguel De Sousa Alves (GSK group of companies). Honoraria Mario Campone (Novartis, Servier, Menar). Consultant or advisory role Mario Campone (Novartis, Servier, Menar), Jean-Luc Canon (GSK). Speakers’ bureau Gilles Romieu (GSK, Roche), Mario Campone (Novartis). Research funding Mario Campone’s institution (Novartis). Expert testimony: Gilles Romieu (Roche). Travel, accommodations, expenses Gilles Romieu (Amgen), Mario Campone (Novartis).
Funding
This work was supported by GlaxoSmithKline Biologicals SA that was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data and the corresponding author was responsible for submission of the publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Giuseppe Curigliano, Lionel Duck, Célia Roemer-Becuwe, Mario Roselli, Silvia Neciosup, Thierry Dorval declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Curigliano, G., Romieu, G., Campone, M. et al. A phase I/II trial of the safety and clinical activity of a HER2-protein based immunotherapeutic for treating women with HER2-positive metastatic breast cancer. Breast Cancer Res Treat 156, 301–310 (2016). https://doi.org/10.1007/s10549-016-3750-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3750-y