Abstract
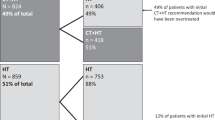
The 21-gene recurrence score (RS) assay (Oncotype DX™) predicts the likelihood of breast cancer recurrence and chemotherapy responsiveness. The aims of this study were to describe temporal trends in assay usage, to investigate factors associated with the receipt of the assay and to determine how the assay is associated with treatment decisions. Random samples of stage I–II female breast cancer patients diagnosed in 2004, 2005 and 2010 as reported to the National Cancer Institute’s Surveillance Epidemiology and End Results program were included. Among women diagnosed in 2010 with estrogen receptor positive (ER+), lymph node-negative (LN−) tumors, factors associated with receipt of the assay were identified and the likelihood of chemotherapy by RS was estimated. Assay usage increased over time (ER+/LN−:8.0–27.0 %, p < 0.01; ER+/LN+: 2.0–15.7 %, p = 0.09; ER−: 0.2–1.7 %, p < 0.01) from 2005 to 2010. Receipt of the assay was associated with younger age, lower area income and tumor characteristics. Among women in the low (RS < 18) and high risk (RS > 30) categories, 3.3 and 95.9 % received chemotherapy, respectively. Within the intermediate risk group the receipt of chemotherapy varied: 12.8 % (RS: 18–19), 35.0 % (RS: 20–23) and 84.0 % (RS: 24–30). During the study years, assay usage increased among women for whom the assay is and is not guideline recommended. Factors such as insurance and race/ethnicity do not appear to be associated with the receipt of the assay. The RS, as determined broadly via three categories and within the intermediate risk group, does appear to influence chemotherapy decisions.
Similar content being viewed by others
References
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 18 Regs Research Data+Hurricane Katrina Impacted Louisiana Cases, Nov 2012 Sub (2000–2010) Katrina/Rita population adjustment: linked to county attributes—total U.S., 1969–2011 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. Released on April 2013, Submitted on November 2012
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826
Eifel P, Axelson JA, Costa J, Crowley J, Curran WJ Jr, Deshler A, Fulton S, Hendricks CB, Kemeny M, Kornblith AB et al (2001) National institutes of health consensus development conference statement: adjuvant therapy for breast cancer. J Natl Cancer Inst 93:979–989
Baker J (2007) Genomic health, Inc. Pharmacogenomics 8(4):397–399
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726–3734
Toi M, Iwata H, Yamanaka T, Masuda N, Ohno S, Nakamura S, Nakayama T, Kashiwaba M, Kamigaki S, Kuroi K (2010) Clinical significance of the 21-gene signature (Oncotype DX) in hormone receptor-positive early stage primary breast cancer in the Japanese population. Cancer 116(13):3112–3118
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25(33):5287–5312
National Comprehansive Cancer Network. NCCN Clinical practice guidelines in oncology: breast cancer. V 1.2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#breast Accessed Nov 2013
Goldstein LJ, Gray R, Badve S, Childs BH, Yoshizawa C, Rowley S, Shak S, Baehner FL, Ravdin PM, Davidson NE et al (2008) Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol 26:4063–4071
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE et al (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11:55–65
Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, Quinn E, Dunbier A, Baum M, Buzdar A et al (2010) Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 28:1829–1834
ClinicalTrials.gov. Tamoxifen citrate, Letrozole, anastrozole, or exemestane with or without chemotherapy in treating patients with invasive RxPONDER breast cancer. http://clinicaltrials.gov/ct2/show/NCT01272037?term=RxPONDER&rank=1 Accessed May 2014
Haas JS, Liang SY, Hassett MJ, Shiboski S, Elkin EB, Phillips KA (2011) Gene expression profile testing for breast cancer and the use of chemotherapy, serious adverse effects, and costs of care. Breast Cancer Res Treat 130(2):619–626
Hassett MJ, Silver SM, Hughes ME, Blayney DW, Edge SB, Herman JG, Hudis CA, Marcom PK, Pettinga JE, Share D et al (2012) Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol 30(18):2218–2226
Lund MJ, Mosunjac M, Davis KM, Gabram-Mendola S, Rizzo M, Bumpers HL, Hearn S, Zelnak A, Styblo T, O’Regan RM (2012) 21-Gene recurrence scores: racial differences in testing, scores, treatment, and outcome. Cancer 118(3):788–796
Chen C, Dhanda R, Tseng WY, Forsyth M, Patt DA (2013) Evaluating use characteristics for the oncotype dx 21-gene recurrence score and concordance with chemotherapy use in early-stage breast cancer. J Oncol Pract/Am Soc Clin Oncol 9(4):182–187
DeFrank JT, Salz T, Reeder-Hayes K, Brewer NT (2013) Who gets genomic testing for breast cancer recurrence risk? Public Health Genom 16(5):215–222
Carlson JJ, Roth JA (2013) The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat 141(1):13–22
Kelly CM, Krishnamurthy S, Bianchini G, Litton JK, Gonzalez-Angulo AM, Hortobagyi GN, Pusztai L (2010) Utility of oncotype DX risk estimates in clinically intermediate risk hormone receptor-positive, HER2-normal, grade II, lymph node-negative breast cancers. Cancer 116(22):5161–5167
National Cancer Institute. SEER registry groupings for analyses. http://seer.cancer.gov/registries/terms.html Accessed 05/07/2013
Breast (2010) In: Edge SB, Byrd DR, Compton CC et al., (eds) AJCC cancer staging manual 7th ed, Springer, New York, pp 347–376. http://www.cancer.gov/cancertopics/pdq/treatment/breast/healthprofessional/page3
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
National Cancer Institute. Surveillance, epidemiology, and end results program (2014) Race recode changes. http://seer.cancer.gov/seerstat/variables/seer/race_ethnicity/ Accessed Aug 2014
American Hospital Association: American Hospital Association Annual Survery Database. http://www.aha.org/research/rc/stat-studies/data-and-directories.shtml
Genomic Health (2014) Press release: medicare contractor establishes reimbursement coverage policy for genomic health’s Oncotype DX(TM) breast cancer test. http://investor.genomichealth.com/releaseDetail.cfm?releaseID=184309 Accessed Aug 2014
Genomic Health (2014) Press release: genomic health announces national agreement with Aetna. http://investor.genomichealth.com/releasedetail.cfm?ReleaseID=219910 Accessed Aug 2014
BlueCross BlueShield of Mississippi (2014) Assays of genetic expression in tumor tissue as a technique to determine prognosis in patients with breast cancer. https://www.bcbsms.com/index.php?q=provider-medical-policy-search.html&action=viewPolicy&path=%2Fpolicy%2Femed%2FAssays_of_Genetic_Expression_in_Tumor_Tissue_as_a_Technique_to_Determine_Prognosis_in_Patients_with_Breast_Cancer.html Accessed Aug 2014
BlueCross BlueShield of North Carolina (2014) Assays of genetic expression to determine prognosis of breast cancer. http://www.bcbsnc.com/assets/services/public/pdfs/medicalpolicy/assays_of_genetic_expression_to_determine_prognosis_of_breast_cancer.pdf Accessed Aug 2014
UnitedHealthcare Medicare Advantage Plans (2014) Coverage summary: genetic testing. https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/UnitedHealthcare%20Medicare%20Coverage/Genetic_Testing_SH_Ovations.pdf. Accessed Aug 2014
UnitedHealthcare. Molecular pathology/molecular diagnostics/genetic testing. https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Main%20Menu/Tools%20&%20Resources/Policies%20and%20Protocols/Medicare%20Advantage%20Reimbursement%20Policies/M/MolecularGeneticTest_01252013.pdf. Accessed Aug 2014
Genomic Health, Inc. (2013) Oncotype Dx FAQs: http://www.mybreastcancertreatment.org/en-US/AboutOncotypeDX/OncotypeDXFAQs.aspx#fbac3992-b9cd-489a-a325-9e84bb78f9af Accessed Dec 2013
Zujewski JA, Kamin L (2008) Trial assessing individualized options for treatment for breast cancer: the TAILORx trial. Future Oncol 4(5):603–610
ClinicalTrials.gov. (2013) Hormone therapy with or without combination chemotherapy in treating women who have undergone surgery for node-negative breast cancer (The TAILORx Trial) http://clinicaltrials.gov/show/NCT00310180 Accessed Dec 2013
Acknowledgments
This work was supported by National Cancer Institute contracts: HHSN261201000024C; HHSN261201000025C, HHSN261201000032C, HHSN261201000027C, HHSN261201000026C, HHSN261201000140C, HHSN261201000037C, HHSN261201000033C, HHSN261201000034C, HHSN261201000035C, HHSN261201000029C, HHSN261201000031C, HHSN261201000028C, and HHSN261201000030C. The authors have disclosed that they have no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors. This article was produced by employees of the US government as part of their official duties and, as such, is in the public domain in the United States of America. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Enewold, L., Geiger, A.M., Zujewski, J. et al. Oncotype Dx assay and breast cancer in the United States: usage and concordance with chemotherapy. Breast Cancer Res Treat 151, 149–156 (2015). https://doi.org/10.1007/s10549-015-3366-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3366-7