Abstract
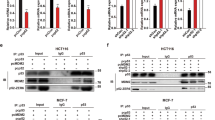
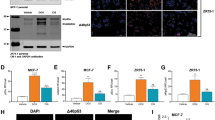
Triple-negative breast cancer (TNBC), the most aggressive breast cancer subtype, occurs in younger women and is associated with poor prognosis. Gain-of-function mutations in TP53 are a frequent occurrence in TNBC and have been demonstrated to repress apoptosis and up-regulate cell cycle progression. Even though TNBC responds to initial chemotherapy, resistance to chemotherapy develops and is a major clinical problem. Tumor recurrence eventually occurs and most patients die from their disease. An urgent need exists to identify molecular-targeted therapies that can enhance chemotherapy response. In the present study, we report that targeting PELP1, an oncogenic co-regulator molecule, could enhance the chemotherapeutic response of TNBC through the inhibition of cell cycle progression and activation of apoptosis. We demonstrate that PELP1 interacts with MTp53, regulates its recruitment, and alters epigenetic marks at the target gene promoters. PELP1 knockdown reduced MTp53 target gene expression, resulting in decreased cell survival and increased apoptosis upon genotoxic stress. Mechanistic studies revealed that PELP1 depletion contributes to increased stability of E2F1, a transcription factor that regulates both cell cycle and apoptosis in a context-dependent manner. Further, PELP1 regulates E2F1 stability in a KDM1A-dependent manner, and PELP1 phosphorylation at the S1033 residue plays an important role in mediating its oncogenic functions in TNBC cells. Accordingly, depletion of PELP1 increased the expression of E2F1 target genes and reduced TNBC cell survival in response to genotoxic agents. PELP1 phosphorylation was significantly greater in the TNBC tumors than in the other subtypes of breast cancer and in the normal tissues. These findings suggest that PELP1 is an important molecular target in TNBC, and that PELP1-targeted therapies may enhance response to chemotherapies.






Similar content being viewed by others
References
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 109(9):1721–1728
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13(15 Pt 1):4429–4434
Liedtke C, Mazouni C, Hess KR et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26(8):1275–1281
Sorlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U.S.A 98(19):10869–10874
Lehmann BD, Bauer JA, Chen X et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121(7):2750–2767
Shah SP, Roth A, Goya R et al (2012) The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 486(7403):395–399
Wright JD, Lim C (2007) Mechanism of DNA-binding loss upon single-point mutation in p53. J Biosci 32(5):827–839
Oren M, Rotter V (2010) Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol 2(2):a001107
Xu J, Reumers J, Couceiro JR et al (2011) Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat Chem Biol 7(5):285–295
Di AS, Strano S, Emiliozzi V et al (2006) Gain of function of mutant p53: the mutant p53/NF-Y protein complex reveals an aberrant transcriptional mechanism of cell cycle regulation. Cancer Cell 10(3):191–202
DeGregori J, Johnson DG (2006) Distinct and overlapping roles for E2F family members in transcription, proliferation and apoptosis. Curr Mol Med 6(7):739–748
Engelmann D, Putzer BM (2012) The dark side of E2F1: in transit beyond apoptosis. Cancer Res 72(3):571–575
Phillips AC, Vousden KH (2001) E2F-1 induced apoptosis. Apoptosis 6(3):173–182
Lin WC, Lin FT, Nevins JR (2001) Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev 15(14):1833–1844
Pediconi N, Ianari A, Costanzo A et al (2003) Differential regulation of E2F1 apoptotic target genes in response to DNA damage. Nat Cell Biol 5(6):552–558
Xie Q, Bai Y, Wu J et al (2011) Methylation-mediated regulation of E2F1 in DNA damage-induced cell death. J Recept Signal Transduction Res 31(2):139–146
Hershko T, Ginsberg D (2004) Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J Biol Chem 279(10):8627–8634
Tonsing-Carter E, Shannon HE, Bailey BJ, Mayo LD, Pollok KE (2013) Blockade of MDM2-mediated signaling in context of DNA damage increases E2F1 expression and enhances cell death in triple-negative breast cancer cells. Cancer Research. 279(10):8627–8634
Gonugunta VK, Miao L, Sareddy GR et al (2014) The social network of PELP1 and its implications in breast and prostate cancers. Endocr Relat Cancer 21(4):T79–T86
Girard BJ, Daniel AR, Lange CA, Ostrander JH (2014) PELP1: a review of PELP1 interactions, signaling, and biology. Mol Cell Endocrinol 382(1):642–651
Habashy HO, Powe DG, Rakha EA et al (2010) The prognostic significance of PELP1 expression in invasive breast cancer with emphasis on the ER-positive luminal-like subtype. Breast Cancer Res Treat 120(3):603–612
Roy S, Chakravarty D, Cortez V et al (2012) Significance of PELP1 in ER-negative breast cancer metastasis. Mol Cancer Res 10(1):25–33
Nair BC, Nair SS, Chakravarty D et al (2010) Cyclin-dependent kinase-mediated phosphorylation plays a critical role in the oncogenic functions of PELP1. Cancer Res 70(18):7166–7175
Nair BC, Krishnan SR, Sareddy GR et al (2014) Proline, glutamic acid and leucine-rich protein-1 is essential for optimal p53-mediated DNA damage response. Cell Death Differ 21(9):1409–1418
Mann M, Cortez V, Vadlamudi R (2013) PELP1 oncogenic functions involve CARM1 regulation. Carcinogenesis 34(7):1468–1475
Bararia D, Trivedi AK, Zada AA et al (2008) Proteomic identification of the MYST domain histone acetyltransferase TIP60 (HTATIP) as a co-activator of the myeloid transcription factor C/EBPalpha. Leukemia 22(4):800–807
Bartek J, Iggo R, Gannon J, Lane DP (1990) Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene 5(6):893–899
Nigro JM, Baker SJ, Preisinger AC et al (1989) Mutations in the p53 gene occur in diverse human tumour types. Nature 342(6250):705–708
Mann M, Cortez V, Vadlamudi RK (2011) Epigenetics of estrogen receptor signaling: role in hormonal cancer progression and therapy. Cancers (Basel) 3(3):1691–1707
Sterner DE, Berger SL (2000) Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev 64(2):435–459
Bossi G, Lapi E, Strano S et al (2006) Mutant p53 gain of function: reduction of tumor malignancy of human cancer cell lines through abrogation of mutant p53 expression. Oncogene 25(2):304–309
Lim LY, Vidnovic N, Ellisen LW, Leong CO (2009) Mutant p53 mediates survival of breast cancer cells. Br J Cancer 101(9):1606–1612
Kontaki H, Talianidis I (2010) Lysine methylation regulates E2F1-induced cell death. Mol Cell 39(1):152–160
Nair SS, Nair BC, Cortez V et al (2010) PELP1 is a reader of histone H3 methylation that facilitates oestrogen receptor-alpha target gene activation by regulating lysine demethylase 1 specificity. EMBO Rep 11(6):438–444
Ueda R, Suzuki T, Mino K et al (2009) Identification of cell-active lysine specific demethylase 1-selective inhibitors. J Am Chem Soc 131(48):17536–17537
Boohaker RJ, Cui X, Stackhouse M, Xu B (2013) ATM-mediated Snail Serine 100 phosphorylation regulates cellular radiosensitivity. Radiother Oncol 108(3):403–408
Sun M, Guo X, Qian X et al (2012) Activation of the ATM-Snail pathway promotes breast cancer metastasis. J Mol Cell Biol 4(5):304–315
Bertheau P, Lehmann-Che J, Varna M et al (2013) p53 in breast cancer subtypes and new insights into response to chemotherapy. Breast 22(Suppl 2):S27–S29
Dobes P, Podhorec J, Coufal O et al (2014) Influence of mutation type on prognostic and predictive values of TP53 status in primary breast cancer patients. Oncol Rep 32:1695–1702
Acknowledgments
This study was supported by the NIH/NCI Grant CA095681 (RKV); CPRIT grant DP150096 (RKV; GR); CPRIT pre-doctoral fellow ship grant RP140105 (SK); CPRIT post-doctoral fellowship grant RP140105 (GRS); and the Cancer Therapy and Research Center at the University of Texas Health Science Center at San Antonio through the NCI Cancer Center Support Grant P30CA054174-17.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishnan, S.R., Nair, B.C., Sareddy, G.R. et al. Novel role of PELP1 in regulating chemotherapy response in mutant p53-expressing triple negative breast cancer cells. Breast Cancer Res Treat 150, 487–499 (2015). https://doi.org/10.1007/s10549-015-3339-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3339-x