Abstract
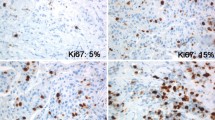
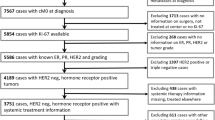
The purpose of this study is to determine the prognostic role of Ki67 evaluated in relapse biopsies from patients with metastatic breast cancer (MBC). Two hundred and ten patients diagnosed with MBC in Stockholm, Sweden between 1998 and 2009 and with Ki67 assessed at time of first systemic relapse (mKi67) were retrospectively identified and divided into two groups according to mKi67 fraction (low ≤20 %, high >20 %). Post-relapse survival was compared between the groups using Kaplan–Meier and Cox regression methods. Death rate as function of continuous mKi67 was also evaluated. Furthermore, the prognostic role of intra-individual change in Ki67 between primary tumor and matched metastasis was explored by Kaplan–Meier plots. One hundred and twenty-five patients had low and 85 had high mKi67. Median survival was 25 and 17 months in low- and high-mKi67 group, respectively [hazard ratio (HR) 0.69, 95 % confidence intervals (CI) 0.51–0.92, P = 0.01]. In a multivariate model adjusted for prognostic confounders, low-mKi67 showed a non-significant trend toward better survival (HR 0.85, 95 %CI 0.62–1.16, P = 0.30). Nevertheless, mKi67 independently correlated with survival when compared with primary tumor proliferation (HR 0.56, 95 %CI 0.38–0.81, P = 0.002). The 2-year death rate steeply increased as mKi67 increased. Moreover, the change from high in primary tumor to low in metastasis significantly correlated with longer survival when compared with stable Ki67 levels (HR 0.48, 95 %CI 0.31–0.76, P = 0.002). In this cohort of MBC patients, mKi67 inversely but not independently correlated with survival. However, a significant association between mKi67 and survival was shown regardless of primary tumor proliferation.



Similar content being viewed by others
Abbreviations
- CI:
-
Confidence intervals
- EBC:
-
Early breast cancer
- ER:
-
Estrogen receptor
- FNAC:
-
Fine-needle aspiration cytology
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hazard ratio
- MBC:
-
Metastatic breast cancer
- mKi67:
-
Ki67 measured in metastasis
- OS:
-
Overall survival
- pKi67:
-
Ki67 measured in primary tumor
- PR:
-
Progesterone receptor
- RFI:
-
Recurrence-free interval
- TMAs:
-
Tissue microarray sections
References
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133:1710–1715
de Azambuja E, Cardoso F, De Castro G, Colozza M Jr, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M (2007) Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 96:1504–1513
Hugh J, Hanson J, Cheang MC, Nielsen TO, Perou CM, Dumontet C, Reed J, Krajewska M, Treilleux I, Rupin M et al (2009) Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol 27:1168–1176
Penault-Llorca F, Andre F, Sagan C, Lacroix-Triki M, Denoux Y, Verriele V, Jacquemier J, Baranzelli MC, Bibeau F, Antoine M et al (2009) Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27:2809–2815
Viale G, Giobbie-Hurder A, Regan MM, Coates AS, Mastropasqua MG, Dell’Orto P, Maiorano E, MacGrogan G, Braye SG, Ohlschlegel C et al (2008) Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol 26:5569–5575
Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills M, Ashley S et al (2005) Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer–a study from the IMPACT trialists. J Clin Oncol 23:2477–2492
Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills M et al (2006) Proliferation and apoptosis as markers of benefit in neoadjuvant endocrine therapy of breast cancer. Clin Cancer Res 12:1024s–1030s
Delpech Y, Wu Y, Hess KR, Hsu L, Ayers M, Natowicz R, Coutant C, Rouzier R, Barranger E, Hortobagyi GN et al (2012) Ki67 expression in the primary tumor predicts for clinical benefit and time to progression on first-line endocrine therapy in estrogen receptor-positive metastatic breast cancer. Breast Cancer Res Treat 135:619–627
Anderson H, Hills M, Zabaglo L, A’Hern R, Leary AF, Haynes BP, Smith IE, Dowsett M (2011) Relationship between estrogen receptor, progesterone receptor, HER-2 and Ki67 expression and efficacy of aromatase inhibitors in advanced breast cancer. Ann Oncol 22:1770–1776
Thompson AM, Jordan LB, Quinlan P, Anderson E, Skene A, Dewar JA, Purdie CA (2010) Breast recurrence in tissues study G: prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the breast recurrence in tissues study (BRITS). Breast Cancer Res 12:R92
Amir E, Miller N, Geddie W, Freedman O, Kassam F, Simmons C, Oldfield M, Dranitsaris G, Tomlinson G, Laupacis A et al (2012) Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol 30:587–592
Foukakis T, Astrom G, Lindstrom L, Hatschek T, Bergh J (2012) When to order a biopsy to characterise a metastatic relapse in breast cancer. Ann Oncol 23(Suppl 10):x349–x353
Lindstrom LS, Karlsson E, Wilking UM, Johansson U, Hartman J, Lidbrink EK, Hatschek T, Skoog L, Bergh J (2012) Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol 30:2601–2608
Amir E, Clemons M, Clemons M, Purdie CA, Miller N, Quinlan P, Geddie W, Coleman RE, Freedman OC, Jordan LB, Thompson AM (2012) Tissue confirmation of disease recurrence in breast cancer patients: pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat Rev 38:708–714
Zajicek J (1974) Aspiration biopsy cytology: Part 1. Cytology of supradiaphragmatic organs. Monogr Clin Cytol 4:131–135
Skoog L, Rutqvist LE, Wilking N (1992) Analysis of hormone receptors and proliferation fraction in fine-needle aspirates from primary breast carcinomas during chemotherapy or tamoxifen treatment. Acta Oncol 31:139–141
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53(282):457–481
Cox DR (1972) Regression models and life-tables. J R Stat Soc Ser B 34(2):187–220
Hayes DF, Ethier S, Lippman ME (2006) New guidelines for reporting of tumor marker studies in breast cancer research and treatment: REMARK. Breast Cancer Res Treat 100:237–238
Polley MY, Leung SC, McShane LM, Gao D, Hugh JC, Mastropasqua MG, Viale G, Zabaglo LA, Penault-Llorca F, Bartlett JM, et al (2013) An international Ki67 reproducibility study. J Natl Cancer Inst 105:1897–1906
Luporsi E, Andre F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, Tubiana-Mathieu N, Sigal-Zafrani B, Arnould L, Gompel A et al (2012) Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat 132:895–915
Foukakis T, Fornander T, Lekberg T, Hellborg H, Adolfsson J, Bergh J (2011) Age-specific trends of survival in metastatic breast cancer: 26 years longitudinal data from a population-based cancer registry in Stockholm, Sweden. Breast Cancer Res Treat 130:553–560
Urruticoechea A, Smith IE, Dowsett M (2005) Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 23:7212–7220
Skoog L, Tani E (2011) Immunocytochemistry: an indispensable technique in routine cytology. Cytopathology 22:215–229
Altman DG, Lausen B, Sauerbrei W, Schumacher M (1994) Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 86:829–835
Denkert C, Loibl S, Muller BM, Eidtmann H, Schmitt WD, Eiermann W, Gerber B, Tesch H, Hilfrich J, Huober J, et al (2013) Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol 24:2786–2793
Acknowledgments
We would like to thank Dr. Ulla Wilking for her help in data collection. This Research Project was supported by the European Society of Medical Oncology (ESMO) “Translational Research Fellowship” to Dr. Falato, the “Umberto Veronesi” Foundation, the Breast Cancer Theme Center (BRECT) at Karolinska Institutet, the Swedish Cancer Foundation, the Cancer Society in Stockholm, the Swedish Research Council, and the Stockholm County Council. Any views, opinions, findings, conclusions, or recommendations expressed in this material are those solely of the authors and do not necessarily reflect those of the above-mentioned organizations.
Conflict of interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Falato, C., Lorent, J., Tani, E. et al. Ki67 measured in metastatic tissue and prognosis in patients with advanced breast cancer. Breast Cancer Res Treat 147, 407–414 (2014). https://doi.org/10.1007/s10549-014-3096-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3096-2