Abstract
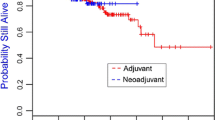
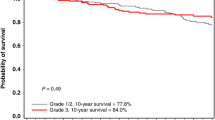
The majority of breast cancers developing in BRCA1 mutation carriers are triple negative breast cancers (TNBC), an aggressive subtype that accounts for 15–20 % of sporadic breast cancer. We compare the clinical outcome and sites of relapse of TNBC in BRCA1 mutation carriers and non-carriers who received adjuvant chemotherapy. Women with stage I–III TNBC who had BRCA1 testing within 36 months of diagnosis and received adjuvant chemotherapy were identified from clinical databases at two academic institutions. Sites of relapse, freedom from distant metastasis (FFDM), and breast cancer-specific survival (BCSS) were determined. RCA1 carriers (n = 89) were significantly younger at diagnosis (P < 0.0001) than non-carriers (n = 175). FFDM at 5 years was 80.5 % for carriers and 76.9 % for non-carriers; with median follow-up of 55 months, hazard ratio (HR) was 0.90, P = 0.71. Sites of recurrence, including brain, did not differ significantly. BCSS at 5 years was 88.1 % for carriers and 81.4 % for non-carriers; HR 0.60; P = 0.15 at 55 months follow-up. BRCA1 carriers who underwent oophorectomy had a significantly lower rate of death from TNBC, with an adjusted HR of 0.30 (95 % CI 0.10–0.94). Adjusting for age, oophorectomy, and prophylactic mastectomy, BRCA1 mutation status was not an independent predictor of survival (HR 2.1; P = 0.13). BRCA1 mutation carriers with TNBC had similar survival rates and sites of recurrence to non-carriers after treatment with conventional chemotherapy. Carriers who underwent oophorectomy had a significantly lower rate of breast cancer-related death; this finding should be studied further in all women with TNBC.


Similar content being viewed by others
References
Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong YN, Blayney DW, Niland JC, Winer EP, Weeks JC (2012) Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118(22):5463–5472. doi:10.1002/cncr.27581
Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, Ramus SJ, Spurdle A, Robson M, Sherman M, Mulligan AM, Couch FJ, Engel C, McGuffog L, Healey S, Sinilnikova OM, Southey MC, Terry MB, Goldgar D, O’Malley F, John EM, Janavicius R, Tihomirova L, Hansen TV, Nielsen FC, Osorio A, Stavropoulou A, Benitez J, Manoukian S, Peissel B, Barile M, Volorio S, Pasini B, Dolcetti R, Putignano AL, Ottini L, Radice P, Hamann U, Rashid MU, Hogervorst FB, Kriege M, van der Luijt RB, Peock S, Frost D, Evans DG, Brewer C, Walker L, Rogers MT, Side LE, Houghton C, Weaver J, Godwin AK, Schmutzler RK, Wappenschmidt B, Meindl A, Kast K, Arnold N, Niederacher D, Sutter C, Deissler H, Gadzicki D, Preisler-Adams S, Varon-Mateeva R, Schonbuchner I, Gevensleben H, Stoppa-Lyonnet D, Belotti M, Barjhoux L, Isaacs C, Peshkin BN, Caldes T, de la Hoya M, Canadas C, Heikkinen T, Heikkila P, Aittomaki K, Blanco I, Lazaro C, Brunet J, Agnarsson BA, Arason A, Barkardottir RB, Dumont M, Simard J, Montagna M, Agata S, D’Andrea E, Yan M, Fox S, Rebbeck TR, Rubinstein W, Tung N, Garber JE, Wang X, Fredericksen Z, Pankratz VS, Lindor NM, Szabo C, Offit K, Sakr R, Gaudet MM, Singer CF, Tea MK, Rappaport C, Mai PL, Greene MH, Sokolenko A, Imyanitov E, Toland AE, Senter L, Sweet K, Thomassen M, Gerdes AM, Kruse T, Caligo M, Aretini P, Rantala J, von Wachenfeld A, Henriksson K, Steele L, Neuhausen SL, Nussbaum R, Beattie M, Odunsi K, Sucheston L, Gayther SA, Nathanson K, Gross J, Walsh C, Karlan B, Chenevix-Trench G, Easton DF, Antoniou AC (2012) Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev 21(1):134–147. doi:10.1158/1055-9965.EPI-11-0775
Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton JK, Potter J, Lanchbury JS, Stemke-Hale K, Hennessy BT, Arun BK, Hortobagyi GN, Do KA, Mills GB, Meric-Bernstam F (2011) Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res 17(5):1082–1089. doi:10.1158/1078-0432.CCR-10-2560
Rummel S, Varner E, Shriver CD, Ellsworth RE (2013) Evaluation of BRCA1 mutations in an unselected patient population with triple-negative breast cancer. Breast Cancer Res Treat 137(1):119–125. doi:10.1007/s10549-012-2348-2
Hicks DG, Short SM, Prescott NL, Tarr SM, Coleman KA, Yoder BJ, Crowe JP, Choueiri TK, Dawson AE, Budd GT, Tubbs RR, Casey G, Weil RJ (2006) Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol 30(9):1097–1104. doi:10.1097/01.pas.0000213306.05811.b9
Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277. doi:10.1200/JCO.2009.25.9820
Lee LJ, Alexander B, Schnitt SJ, Comander A, Gallagher B, Garber JE, Tung N (2011) Clinical outcome of triple negative breast cancer in BRCA1 mutation carriers and noncarriers. Cancer 117(14):3093–3100. doi:10.1002/cncr.25911
Bayraktar S, Gutierrez-Barrera AM, Liu D, Tasbas T, Akar U, Litton JK, Lin E, Albarracin CT, Meric-Bernstam F, Gonzalez-Angulo AM, Hortobagyi GN, Arun BK (2011) Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Res Treat 130(1):145–153. doi:10.1007/s10549-011-1711-z
Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L, Litton JK, Valero V, Meric-Bernstam F, Hortobagyi GN, Albarracin C (2011) Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol 29(28):3739–3746. doi:10.1200/JCO.2011.35.2682
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100(14):8418–8423. doi:10.1073/pnas.09326921000932692100
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM (2004) Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 10(16):5367–5374. doi:10.1158/1078-0432.CCR-04-022010/16/5367
Turner NC, Reis-Filho JS (2006) Basal-like breast cancer and the BRCA1 phenotype. Oncogene 25(43):5846–5853. doi:10.1038/sj.onc.1209876
Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, Savage K, Gillett CE, Schmitt FC, Ashworth A, Tutt AN (2007) BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene 26(14):2126–2132. doi:10.1038/sj.onc.1210014
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Investig 121(7):2750–2767. doi:10.1172/JCI45014
Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD, Pietenpol JA, Hortobagyi GN, Symmans WF, Ueno NT (2013) Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res 19(19):5533–5540. doi:10.1158/1078-0432.CCR-13-0799
Mulligan JM, Hill LA, Deharo S, Irwin G, Boyle D, Keating KE, Raji OY, McDyer FA, O’Brien E, Bylesjo M, Quinn JE, Lindor NM, Mullan PB, James CR, Davison TS, Walker SM, Kerr P, James J, Proutski V, Salto-Tellez M, Johnston PG, Couch FJ, Paul Harkin D, Kennedy RD (2014) Identification and validation of an anthracycline/cyclophosphamide-based chemotherapy response assay in breast cancer. J Natl Cancer Inst 106(1):djt335. doi:10.1093/jnci/djt335
Von Minckwitz G SA, Salat C et al (2013) A randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto). J Clin Oncol 31
Sikov WM BD, Perou CM et al (2013) Impact of the addition of carboplatin (Cb) and/or bevacizumab (B) to neoadjuvant weekly paclitaxel (P) followed by dose-dense AC on pathologic complete response (pCR) rates in triple-negative breast cancer (TNBC): CALGB 40603 (Alliance). In: The 2013 San Antonio Breast Cancer Symposium, December 10–14 2013, Abstract S5-01
Rugo HS, Olopade O, DeMichele A et al (2013) Veliparib/carboplatin plus standard neoadjuvant therapy for high-risk breast cancer: first efficacy results from the I-SPY 2 TRIAL. Presented at the 36th annual San Antonio breast cancer symposium, December 10–14 2013, San Antonio, TX, Abstract S5-02
Telli ML, Jensen K, Kurian AW et al (2013) PrECOG 0105: final efficacy results from a phase II study of gemcitabine and carboplatin plus iniparib (BSI201) as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer. ASCO Annual Meeting, June 3 2013, Abstract 1003
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, Mierzwa T, Szwiec M, Wisniowski R, Siolek M, Dent R, Lubinski J, Narod S (2010) Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol 28(3):375–379. doi:10.1200/JCO.2008.20.7019
Gronwald JBT, Huzarski T, Dent R, Bielick V, Zuziak D, Wisniowski R, Lubinski J, Narod S (2009) Neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. J Clin Oncol 27(15s):502
Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, Fatima A, Gelman RS, Ryan PD, Tung NM, De Nicolo A, Ganesan S, Miron A, Colin C, Sgroi DC, Ellisen LW, Winer EP, Garber JE (2010) Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol 28(7):1145–1153. doi:10.1200/JCO.2009.22.4725
National Cancer Institute. Clinical Trials (PDQ®). http://www.cancer.gov/clinicaltrials/search/view?cdrid=739199&version=HealthProfessional&protocolsearchid=12620420. Accessed 14 April 2014
Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler RK, Wardley A, Mitchell G, Earl H, Wickens M, Carmichael J (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 376(9737):235–244. doi:10.1016/S0140-6736(10)60892-6
Gelmon KA, Hirte HW, Robidoux A, Tonkin KS, Tischkowitz M, Swenerton K, Huntsman D, Carmichael J, Macpherson E, Oza AM (2010) A phase II correlative study of olaparib in advanced serous ovarian cancer and triple-negative breast cancer. J Clin Oncol 28(15s):3002
Albiges L, Andre F, Balleyguier C, Gomez-Abuin G, Chompret A, Delaloge S (2005) Spectrum of breast cancer metastasis in BRCA1 mutation carriers: highly increased incidence of brain metastases. Ann Oncol 16(11):1846–1847. doi:10.1093/annonc/mdi351
Sekine M, Yoshihara K, Komata D, Haino K, Nishino K, Tanaka K (2013) Increased incidence of brain metastases in BRCA1-related ovarian cancers. J Obstet Gynaecol Res 39(1):292–296. doi:10.1111/j.1447-0756.2012.01961.x
Faluyi OO, Gourley C, Smyth JF, Faratian D, Williams AR, Rye T, Rye R, Stewart M, Mackean MJ (2010) Higher incidence of isolated brain metastases in ovarian cancer patients with previous early breast cancer. Int J Gynecol Cancer 20(9):1511–1517
Koul A, Loman N, Malander S, Borg A, Ridderheim M (2001) Two BRCA1-positive epithelial ovarian tumors with metastases to the central nervous system: a case report. Gynecol Oncol 80(3):399–402. doi:10.1006/gyno.2000.6085
Gourley C, Michie CO, Roxburgh P, Yap TA, Harden S, Paul J, Ragupathy K, Todd R, Petty R, Reed N, Hayward RL, Mitchell P, Rye T, Schellens JH, Lubinski J, Carmichael J, Kaye SB, Mackean M, Ferguson M (2010) Increased incidence of visceral metastases in Scottish patients with BRCA1/2-defective ovarian cancer: an extension of the ovarian BRCAness phenotype. J Clin Oncol 28(15):2505–2511. doi:10.1200/JCO.2009.25.1082
Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles R, Pichert G, Van t’veer L, Tung N, Weitzel JN, Couch FJ, Rubinstein WS, Ganz PA, Daly MB, Olopade OI, Tomlinson G, Schildkraut J, Blum JL, Rebbeck TR (2010) Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 304(9):967–975. doi:10.1001/jama.2010.1237
Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26(8):1275–1281. doi:10.1200/JCO.2007.14.4147
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13(15 Pt 1):4429–4434. doi:10.1158/1078-0432.CCR-06-3045
Disclosure
This research was supported by grants from the Breast Cancer Research Foundation.
Ethical Standards
All research activities carried out for this study comply with the current laws of the United States of America, where this research took place.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tung, N., Gaughan, E., Hacker, M.R. et al. Outcome of triple negative breast cancer: comparison of sporadic and BRCA1-associated cancers. Breast Cancer Res Treat 146, 175–182 (2014). https://doi.org/10.1007/s10549-014-2995-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2995-6