Abstract
We report prospectively observed risk for breast cancer in breast cancer kindreds without a demonstrable BRCA1/2 mutation. According to family history, the optimal available member(s) of each breast cancer kindred attending our clinic was tested for BRCA mutations. Women in families without a demonstrable BRCA mutation were subjected to annual mammography. BRCA mutations were demonstrated in 496/2,118 (23 %) breast cancer kindreds. In families without a demonstrable BRCA mutation, a total of 3,161 healthy women aged 25–59 years were prospectively followed for 24,808 observation years. Sixty-four cancers were observed, compared to 34.0 expected (p < 0.01), arriving at a 7.9 % cumulative risk at age 60 compared to 4.0 % in the population [relative risk (RR) = 2.0]. Women with one mother or sister affected ≤50 years and with no other close relatives with breast cancer did not have increased risk (0 cancers observed and 0.6 expected at age 40, 11 cancers observed and 7.9 expected at age 60, p > 0.05). Excluding these, cumulative risk at 60 years was 8.8 % (RR = 2.2). The highest cumulative risk at 60 years was 11.4 %, found in families with two cases ≤55 years (RR = 2.8). In breast cancer kindreds without a demonstrable BRCA mutation, the risk for breast cancer in female first degree relatives was about twice the risk in the general population. Women with one early affected relative only did not have increased risk for early onset breast cancer, while those with more than one young affected relative had close to three times population risk.
Similar content being viewed by others
In cancer genetic clinics, genetic counseling based on family history of breast cancer and disclosure of results of genetic testing is the daily routine. In most breast cancer kindreds, no causative genetic mutation is found. Models to predict risk are usually based on retrospective studies of family histories with the intention to select families likely to have causative BRCA1/2 mutations. Because access to genetic testing has been the limiting factor, family history has been used to select families with high probabilities of BRCA mutations for mutation testing. (For an overview see Claus [1] and BOADICEA [2].) How these models arrive at predictive values of risk for breast cancer in women tested to not have a disease-causing mutation, is not based on prospective observations and may be influenced by assumptions. There is limited information based on prospectively observed incidence rates of breast cancer in adult female relatives to breast cancer patients in breast cancer kindreds without a demonstrable genetic cause.
The Inherited Cancer Research Group at The Norwegian Radium Hospital has comprehensive access to the records of families affected by breast cancer. We have previously reported that only 23 % of BRCA1 mutation carriers in a series of incident breast cancers met the family-based criteria used to select a patient for BRCA testing [3]. We here report the prevalence of deleterious BRCA mutations in breast cancer kindreds meeting these criteria in our out patient cancer genetic clinics, and the cumulative incidence rates for breast cancer by age in breast cancer kindreds not having a demonstrable causative mutation.
Materials and methods
Our team has identified families with breast or breast–ovarian cancer for more than 20 years from across Norway. Selection criteria and follow-up protocols were published and became national guidelines [4]. The later international guidelines [5] were compatible with our national guidelines, which we maintained for our continued activity. The criteria were: (1) four cases of breast cancer in the family, (2) two cases = <55 years of age, (3) one case = <60 years of age with bilateral breast cancer, (4) one case with breast and another cancer = <60 years, (5) one case with breast cancer = <50 years, and (6) one case with breast and a first degree relative with ovarian cancer or a woman with both breast and ovarian cancer in the family. In families meeting one or more of the criteria, first degree female relatives of breast or ovarian cancer cases were considered at risk and invited to monitored follow-up. Second degree relatives through males, were offered health surveillance as well, but are not included in this report. An included patient could meet one, some or all these criteria. When an individual came close to the criteria, the team was able to exercise discretion and offer testing and follow-up.
Each patient had genetic counseling at our out patient genetic clinic before inclusion, signed informed consent to genetic testing, and provided a family tree with the details of first and second degree relatives including age, sex, and cancers. In most cases, we obtained medical records to validate all breast cancer cases in the family and invited all living close relatives with any cancer to provide blood samples for genetic testing.
For categorizing all women included according to family history, we excluded all cases prospectively diagnosed during the study. For this report, we initially analyzed all families without a demonstrable BRCA mutation as one group, from now on referred to as "the total series." We decided to analyze four subgroups: (1) at least four breast cancer cases in the family, (2) at least two breast cancer cases = <55 years in the family, (3) one breast cancer case only in the family and that case = <50 years at onset, and (4) kindreds with both breast and ovarian cancer. These four subgroups were selected because the experience was that the groups 1–3 had been the inclusion criteria most often clinically discussed during the years and group 4 might indicate BRCA mutations overlooked by the genetic testing and/or the presence of other genes causing both breast and ovarian cancer not tested for. Also, group 1 was considered indicative of gene(s) with high life-time penetrance, group 2 was considered indicative of highly penetrant gene(s) with early onset of disease, and group 3 had been a frequent clinical problem when a young woman presented herself with a young mother or sister dying or dead from breast cancer. Also, group 3 would give information on recessive inheritance.
The follow-up included annual mammography for women aged 25 or more. While our study was ongoing, biennial mammography screening was offered to all women in the population from 50 to 70 years of age. We then referred most of our patients past 60 years to the population screening. We censored the current study at 60 years of age.
Availability of genetic testing has developed over the years. Initially, we described that the presence of frequent (founder) BRCA1/2 mutations were responsible for the majority of the carriers of breast cancer mutations and all families were tested for these mutations [3]. Later, all kindreds without a demonstrated BRCA1/2 founder mutation were examined by Sanger sequencing and multiplex ligation-dependent probe amplification (MLPA) of BRCA1/2. From the onset, we had stored blood samples and informed consents from all cancer cases available in all families, and from all prospectively detected cases, and we in this way were able to genetically test all prospectively detected cancer cases, including all who had died before testing became available. The timing of the current report reflects that the testing as described below was completed in all families.
-
(1)
Under a hypothesis of dominantly inherited breast or breast–ovarian cancer in the families, all available obligate carriers with breast or ovarian cancer, or affected possible mutation carriers (as, for example, an affected woman having no children), were Sanger sequenced and MLPA tested.
-
(2)
When no causative mutation in the family was found this way, healthy obligate carriers (often males) were Sanger sequenced and MLPA tested.
-
(3)
When a causative BRCA mutation still had not been excluded (typically when an affected mother was dead and unavailable for testing), the individual women at risk were tested for the Norwegian founder mutations and in many cases subjected to Sanger sequencing and MLPA testing as well.
-
(4)
Daughters to affected cases demonstrated to not have a BRCA mutation were not tested unless the family history indicated a possibility of inherited cancer in both the paternal and the maternal lineage. If so, the family was considered two families and steps 1, 2 were conducted in both lineages and including testing of the at-risk daughters to males corresponding to step 3. As mentioned above, these at-risk daughters were not included in the study, if their mothers had not had cancer, but the procedure was part of identifying mutation-carrying kindreds.
-
(5)
All prospectively detected cancers were Sanger sequenced and MLPA tested.
-
(6)
All families in which a pathogenic mutation was found in any member, were excluded from the study.
Families containing cancer cases suggestive of the Li–Fraumeni (SBLA) syndrome [6] or the Cowden syndrome [7] were tested for TP53 and PTEN mutations and the mutation-carrying families were excluded from the present study. Findings in mutation-carrying kindreds have been or will be reported separately.
Follow-up implied referral to mammography at a breast diagnostic center where, in addition, ultrasound, clinical examination, fine needle aspiration cytology, core, and excision biopsy were available without delay when indicated. This report describes the combined results of these diagnostic modalities in a clinical setting and is not an analysis of sensitivities of the different modalities per se to demonstrate cancer. Such analyses would not be meaningful in our clinical series, where the result of the first examination was known to the person interpreting the next examination in each patient.
For the current study, all cases had breast or ovarian cancer prior to inclusion, or cancer demonstrated at the first (prevalence) round, were excluded. All cancers after first control were counted, including interval cancers, without reference to how the cancer was detected. Each woman was censored at the date for breast cancer demonstrated or last examination, whichever came first. One patient was counted once only, without notion of bilateral cancers. No other cancer than breast cancer was scored as an event.
To compare our series with the Norwegian Cancer Registry (www.kreftregisteret.no) as population controls, we copied the cancer registry’s method and categorized the observations into 5-year cohorts to determine the age-specific incidence rates in each age group. Carcinoma in situ was not scored as cancer. All women were scored with respect to age groups for each year they were observed. Differences between expected and observed numbers of breast cancers were considered with χ 2 tests. Annual incidence rates (AIRs) were calculated for each age group separately, and were compared to similar groups from the cancer registry as controls, arriving at standardized incidence ratios (SIRs) with 95 % confidence intervals (CIs). Based on the observed AIRs for each age group, the cumulative incidences at different ages were calculated, starting with cumulative incidence at age 25 years set to 0. Relative cumulative incidence risks (RRs) compared to the population controls were calculated.
The follow-up was censored December 2011. Data were stored and computed inside our medical filing system CGEN [8] and with use of TOAD © and SYSTAT 13 ©. No named data were exported from the medical files. All patients had at least one genetic counseling session, and all genetic testing included written informed consent and were conducted according to national legislation. The study was approved by the Ethical review board (ref S02030) and The Norwegian Data Inspectorate (ref 2001/2988-2). The present report is one in a series to meet the request from The Norwegian Parliament to report the results of our activities.
Results
A total of 7,748 persons were tested for BRCA mutations. Deleterious mutations were found in 496 out of 2,118 (23 %) independent breast cancer kindreds tested.
From families without a demonstrable BRCA mutation (‘the total series’), a total of 3,161 women met the inclusion criteria and were observed for a total of 24,808 years (mean follow-up time 7.9 years). Family data for categorizing into subgroups based on family history was available for 2,962 patients (94 %), and among them 1,742 (59 %) met one or more of the four criteria for subgroups. One-hundred and seventy-two women had both two close relatives with breast cancer = <55 years of age and four or more cases irrespective of age in their families. We found this number insufficient to examine this group separately. The criterion one breast cancer ≤50 years in family only was not overlapping any of the other criteria. Most (615 out of 860 = 72 %) of the cases with ovarian cancer in their families met more than one inclusion criteria. Because no excess of cancers was demonstrated in the breast and ovarian cancer families (Table 1) and because no very young onset breast cancer case was seen in this group (Table 2), no subgroup within this group was analyzed. This left us with the total series and the four subgroups to examine further.
In the total series, 64 breast cancers were demonstrated, compared to 34.0 expected (p < 0.01; Table 1). In families with 4 or more breast cancer cases, 9 cases were prospectively demonstrated, compared to 3.7 expected (p < 0.05). In families with at least two cases ≤55 years at onset, 26 cases were demonstrated, compared to 9.2 expected (p < 0.05). In contrast, there was no excess of breast cancer cases demonstrated neither in women with only one mother or sister having had breast cancer at young age (11 demonstrated compared to 7.8 expected, p > 0.05) nor in the families with both breast and ovarian cancer (14 demonstrated compared to 8.7 expected, p > 0.05). The same differences were seen in all groups, when considering cumulative risk at 50 years of age (Table 1). For both those with only one mother or sister affected at young age, and for those with ovarian cancer in the family, the cumulative risk at age 40 was 0 observed, compared to 0.6 expected.
The AIRs for each age group are given in Table 2. Based on these, cumulative incidences by age were calculated in the total series to 1.0 % at age 40, 3.9 % at age 50, and 7.9 % at age 60, corresponding to RR of 2.9, 2.4, and 2.0, respectively (Table 3).
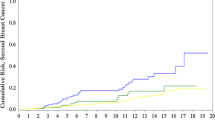
Patients from families with two breast cancer cases = <55 years of age had the highest cumulative incidence rate at all ages, while those with only one early onset cancer in the family had the lowest cumulative incidence rate at all ages. Figure 1 demonstrates the cumulative risk for each year from 25 to 60 years for all groups.
Cumulative risk by age in all cases with familial breast cancer without a demonstrable mutation (Total_series), in women with four or more affected relatives (four cases in family), in women with two or more affected relatives = <55 years (two cases ≤55), in women with both breast and ovarian cancer in the family (ovarian cancer in family), in women where the only affected relative was the mother or one sister = <50 years (one young case ≤50 only in family), and in population (cancer registry as controls)
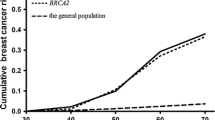
Excluding all women with one relative = <50 years only from the total series, cumulative risks at 40, 50 and 60 years were 1.5, 4.5 and 8.8 %, respectively, corresponding to RR of 2.2 at age 60 years. As shown in Fig. 2, for most ages women in this group had a cumulative incidence similar to that of women 10 years older in the general population.
We found the distribution curve in Fig. 1 compatible with a limited subgroup within the group two cases in family = <55 years having an early onset breast cancer risk, which is compatible with a monogenic factor with high penetrance and early onset.
More than one prospective case was diagnosed in nine families: three cases in three families, two cases in six families. Four of these families had four or more additional breast cancer cases in the family, among which three had two affected ≤55 years of age. All with two breast cancer cases ≤55 years in the families had four additional cases in the family. One of the nine families had one affected additional case only and that one <50 years of age. In seven of the nine families BRCA1/2, haplotyping was possible and undertaken by use of intragenic and flanking markers and no indication of linkage between BRCA1/2 haplotypes and breast cancer was found (data not shown). The nine families were interpreted as in keeping with a theory of highly penetrant inherited factors other than mutated BRCA1/2 genes having caused disease in (some of) the families. Extended genetic testing will be carried out to search for highly penetrant genetic factors in all the prospective cases reported here, pending resources to do so.
The lack of an increased risk to sisters of young onset breast cancer cases was in conflict with expectation if assuming recessive inheritance.
Discussion
Through the current report, we now have empirical figures for breast cancer risk in breast cancer kindreds where a BRCA mutation is not demonstrable: the cumulative incidence rate for breast cancer in breast cancer kindreds without a demonstrable genetic cause was 7.9 % = RR 2.0 at age 60 years. Those having only one early affected first degree relative did not have increased risk for early onset breast cancer. Restricting the analysis to women with two ore more breast cancer cases in the family irrespective of age, an RR at 60 years of 2.2 was obtained, corresponding to women 10 years older in the general population. The highest RR at 60 years in any analyzed subgroup was 2.8.
Sanger sequencing and MLPA testing are insensitive to detect medium-sized deletions [9]. However, the lack of an increased risk for young onset breast cancer in families with both breast and ovarian cancer, indicated that all or close to all families with BRCA mutations had been identified and excluded from the study. Also, this indicated that the excess of early onset breast cancers observed was not caused by other high-penetrant genes for early onset breast and ovarian cancer not tested for. As soon as resources permit all prospectively detected cases will be examined for all genes reportedly associated with breast cancer. If this may not explain the findings, we may sequence all protein-coding exons in all genes in all prospective cancer cases to look for new genes causing breast cancer.
Compared to our series, a report by Metcalfe et al. [10] from North America reported about twice our observed AIRs. Besides their lower number of observation years (9,109 compared to 24,808 in our study), there were methodological differences between the studies: Metcalf et al. used questionnaires and had a mean follow-up time of 6.1 years, implying they had no identified prevalence round and were unable to remove prevalence cancers. We recorded an overall prevalence rate of 0.60 % breast cancers at first planned mammography, which compared with our observed overall AIR of 0.26 % represented 2.3 years cumulative incidence, which is about one third of the observation period reported by Metcalfe et al. We subjected the families to more detailed genetic testing. Metcalfe et al. did not test their prospectively detected cancers for BRCA mutations. We assigned each woman to a 5 year cohort for each year observed. Metcalfe et al. grouped their observations on age at baseline, implying that on average more than half of the observation years in a woman included as belonging to one age group, might have been recorded, when she had become older and actually belonged to an older age group. Also, the higher incidence rates in the younger reported by Metcalfe et al. were in parallel to the higher risk for young BRCA1 mutation carriers in North America as compared to Poland and Norway [11, 12] and may reflect environmentally caused differences between the populations in Europe and North America. The difference between our findings and those of Metcalfe et al. is, however, not significant to the discussion in this report on moving toward personalized medicine. That notion is based on the observations on risk for cancer in mutation carriers, of the much lower risk for cancer in kindreds without a demonstrable mutation, and that validation of family histories in addition to what the patients may tell on-the-fly add little to the risk estimates after testing.
After this study was completed and while the report was written, other reports on findings in breast cancer kindreds without demonstrated mutations have been published: two follow-up studies on high-risk women with MRI (MARIBS in UK and MRISC in Holland [13]) report prospective findings in familial breast cancer, but to which degree the familial breast cancer kindreds actually were BRCA tested is unclear, and the reports were not organized to answer the questions addressed in our study. The same may be noted for two UK studies based on family history of breast cancer [14, 15]. We look forward to see reports from other centers focusing the questions addressed in this paper.
Speculating on the mechanisms having caused our observations, we may mention: besides the notion that the distribution curves may indicate a small subset with not identified highly penetrant genetic factor(s), the findings were as expected if assuming multiple genetic and/or environmental factors having caused the family histories of breast cancer. Non-random mating has been frequent in Norway [3] and may give multifactorially caused clusters of familial cancers with an increased recurrence risk in the next generations compared to random mating. Which, if the degree of inmating declines, will lead to decreased recurrence risk in the families in the future. Multifactorial inheritance may also explain that in those with one early affected first degree relative only, the risk would be but moderately increased and the next affected in the family would be expected to have an age in-between index case and population mean, which was the point estimate observed.
Conclusions
In breast cancer kindreds the presence/absence of a BRCA1/2 mutation is the major determinant of risk for breast cancer. The risk for breast cancer when a pathogenic BRCA mutation is demonstrated is known. We here present the first comprehensive empirical observations on risk for breast cancer in families not having a demonstrable BRCA mutation, which is what most genetic counseling sessions are about when disclosing the results of BRCA testing. In short, women in breast cancer kindreds without a demonstrable BRCA mutation had about twice the risk of women in the population to contract breast cancer at any age, with the notion that having only one early affected mother or sister was not associated with the increased risk for early onset breast cancer and that in kindreds with multiple young cases there may be other high-penetrant risk factors than BRCA mutations to look for.
References
Claus EB (2001) Risk models used to counsel women for breast and ovarian cancer: a guide for clinicians. Fam Cancer 1:197–206
http://www.srl.cam.ac.uk/genepi/boadicea/boadicea_intro.html. Accessed 30 June 2013
Møller P, Hagen AI, Apold J et al (2007) Genetic epidemiology of BRCA mutations-family history detects less than 50 % of the mutation carriers. Eur J Cancer 43(11):1713–1717
Saetersdal A, Dørum A, Heimdal K et al (1996) Inherited predisposition to breast carcinoma. Results of first round examination of 537 women at risk. Anticancer Res 16(4A):1989–1992
Møller P, Evans G, Haites N et al (1999) Guidelines for follow-up of women at high risk for inherited breast cancer: consensus statement from the Biomed 2 Demonstration Programme on Inherited Breast Cancer. Dis Markers 15(1–3):207–211
Birch JM (2004) Genetic predisposition to cancer, pp 141–154. www.arnold-publishers.com
Eng C (2004) Genetic predisposition to cancer, pp 155–166. www.arnold-publishers.com
Møller P, Clark N (2011) CGEN—a Clinical GENetics software application. Hum Mutat 32(5):537–542
Herman S, Varga D, Deissler HL, Kreienberg R, Deissler H (2012) Medium-sized deletion in the BRCA1 gene: limitations of Sanger sequencing and MLPA analyses. Genet Mol Biol 35(1):53–56
Metcalfe KA, Finch A, Poll A et al (2009) Breast cancer risks in women with a family history of breast or ovarian cancer who have tested negative for a BRCA1 or BRCA2 mutation. Br J Cancer 100(2):421–425
Lubinski J, Huzarski T, Byrski T et al (2012) The risk of breast cancer in women with a BRCA1 mutation from North America and Poland. Int J Cancer 131(1):229–234
Møller P, Maehle L, Vabø A, Clark N, Sun P, Narod SA (2013) Age-specific incidence rates for breast cancer in carriers of BRCA1 mutations from Norway. Clin Genet 83(1):88–91
Tilanus-Linthorst MM, Lingsma HF, Evans DG, Thompson D, Kaas R, Manders P, van Asperen CJ, Adank M, Hooning MJ, Kwan Lim GE, Eeles R, Oosterwijk JC, Leach MO, Steyerberg EW (2013) Optimal age to start preventive measures in women with BRCA1/2 mutations or high familial breast cancer risk. Int J Cancer 133(1):156–163
Evans DG, Thomas S, Caunt J, Roberts L, Howell A, Wilson M, Fox R, Sibbering DM, Moss S, Wallis MG, Eccles DM, Duffy S; FH02 Study Group (2014) Mammographic surveillance in women aged 35–39 at enhanced familial risk of breast cancer (FH02). Fam Cancer 13(1):13–21
Evans DG, Ingham S, Dawe S, Roberts L, Lalloo F, Brentnall AR, Stavrinos P, Howell A (2013) Breast cancer risk assessment in 8,824 women attending a family history evaluation and screening programme. Fam Cancer. doi:10.1007/s10689-013-9694-z
Acknowledgments
Thanks to all collaborating research and diagnostic DNA laboratories and breast cancer diagnostic centers engaged in the activities since 1989.
Ethical standards
The activities reported were carried out according to national legislation, and were approved by the Ethical review board (S-02030) and The Norwegian Data Inspectorate (2001/2988-2).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Møller, P., Stormorken, A., Holmen, M.M. et al. The clinical utility of genetic testing in breast cancer kindreds: a prospective study in families without a demonstrable BRCA mutation. Breast Cancer Res Treat 144, 607–614 (2014). https://doi.org/10.1007/s10549-014-2902-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2902-1