Abstract
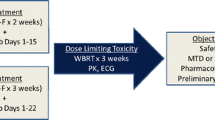
Brain metastases are common in patients with advanced, Human Epidermal Growth Factor Receptor 2 (HER2)-positive breast cancer. We evaluated the maximum tolerated dose (MTD) and feasibility of lapatinib given concurrently with whole brain radiotherapy (WBRT). Eligible patients had (HER2)-positive breast cancer and ≥1 brain metastasis. Patients received lapatinib 750 mg twice on day one followed by 1000, 1250, or 1500 mg once daily. WBRT (37.5 Gy, 15 fractions) began 1–8 days after starting lapatinib. Lapatinib was continued through WBRT. Following WBRT, patients received trastuzumab 2 mg/kg weekly and lapatinib 1000 mg once daily. The regimen would be considered feasible if <3/27 pts treated at the MTD experienced a dose-limiting toxicity (DLT). Thirty-five patients were enrolled; 17 % had central nervous disease (CNS) only. During dose escalation, no patients receiving 1,000 or 1,250 mg and two of five patients receiving 1,500 mg experienced DLTs (grade 3 mucositis and rash). Overall, 7/27 patients at 1,250 mg (MTD) had DLTs: grade 3 rash (n = 2), diarrhea (n = 2), hypoxia (n = 1), and grade 4 pulmonary embolus (n = 2). Among 28 evaluable patients, the CNS objective response rate (ORR) was 79 % [95% confidence interval (CI) 59–92 %] by pre-specified volumetric criteria; 46 % remained progression-free (CNS or non-CNS) at 6 months. The study did not meet the pre-defined criteria for feasibility because of toxicity, although the relationship between study treatment and some DLTs was uncertain. Given the high ORR, concurrent lapatinib-WBRT could still be considered for future study with careful safety monitoring.

Similar content being viewed by others
References
Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, Bunnell C, Rue M, Gelman R, Winer E (2003) Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97:2972–2977
Clayton AJ, Danson S, Jolly S, Ryder WD, Burt PA, Stewart AL, Wilkinson PM, Welch RS, Magee B, Wilson G et al (2004) Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer 91:639–643
Pestalozzi BC, Holmes E, de Azambuja E, Metzger-Filho O, Hogge L, Scullion M, Lang I, Wardley A, Lichinitser M, Sanchez RI et al (2013) CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol 14:244–248
Gori S, Rimondini S, De Angelis V, Colozza M, Bisagni G, Moretti G, Sidoni A, Basurto C, Aristei C, Anastasi P et al (2007) Central nervous system metastases in HER-2 positive metastatic breast cancer patients treated with trastuzumab: incidence, survival, and risk factors. Oncologist 12:766–773
Eichler AF, Kuter I, Ryan P, Schapira L, Younger J, Henson JW (2008) Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer 112:2359–2367
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J et al (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419–425
Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL (2002) Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 21:6255–6263
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B et al (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743
Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J et al (2010) Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, Trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28:1124–1130
Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, Kurek R, Vega-Valle E, Feigenbaum L, Halverson D et al (2007) Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res 67:4190–4198
Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, Liewehr DJ, Steinberg SM, Merino MJ, Rubin SD et al (2008) Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst 100:1092–1103
Kuhn JG, Robbins H, Mehta M, Fine HA, Cloughesy T, Wen PY, Chang S, DeAngelis L, Lieberman F, Reardon D et al (2008) Tumor Sequestration of Lapatinib (NABTC 04-01). Neuro Oncol 10:783 (Abstract ET-05)
Morikawa A, Peereboom D, Smith QR, Thorsheim HR, Lockman PR, Simmons J, Weil RJ, Tabar V, Steeg PS, Seidman A (2013) Clinical evidence for drug penetration of capecitabine and lapatinib uptake in resected brain metastases from women with metastatic breast cancer. Presented at the 2013 American Society of Clinical Oncology Meeting (Chicago, IL). J Clin Oncol 31 (suppl; abstr 514)
Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, Harris GJ, Bullitt E, Van den Abbeele AD, Henson JW et al (2008) Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 26:1993–1999
Zhou H, Kim YS, Peletier A, McCall W, Earp HS, Sartor CI (2004) Effects of the EGFR/HER2 kinase inhibitor GW572016 on EGFR- and HER2-overexpressing breast cancer cell line proliferation, radiosensitization, and resistance. Int J Radiat Oncol Biol Phys 58:344–352
Sartor CI (2004) Mechanisms of disease: radiosensitization by epidermal growth factor receptor inhibitors. Nat Clin Pract Oncol 1:80–87
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J et al (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578
Storniolo AM, Pegram MD, Overmoyer B, Silverman P, Peacock NW, Jones SF, Loftiss J, Arya N, Koch KM, Paul E et al (2008) Phase I dose escalation and pharmacokinetic study of lapatinib in combination with trastuzumab in patients with advanced ErbB2-positive breast cancer. J Clin Oncol 26:3317–3323
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN et al (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–181
Cockrell JR, Folstein MF (1988) Mini-Mental State Examination (MMSE). Psychopharmacol Bull 24:689–692
Folstein MF, Robins LN, Helzer JE (1983) The mini-mental state examination. Arch Gen Psychiatry 40:812
Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA (1995) The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer 75:1151–1161
Osoba D, Aaronson NK, Muller M, Sneeuw K, Hsu MA, Yung WK, Brada M, Newlands E (1996) The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res 5:139–150
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Tsao MN, Lloyd N, Wong RK, Chow E, Rakovitch E, Laperriere N, Xu W, Sahgal A (2012) Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev 4:CD003869
Tsao M, Xu W, Sahgal A (2012) A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer 118:2486–2493
Viani GA, Manta GB, Fonseca EC, De Fendi LI, Afonso SL, Stefano EJ (2009) Whole brain radiotherapy with radiosensitizer for brain metastases. J Exp Clin Cancer Res 28:1
Suh J, et al. (2008) Results of the Phase III ENRICH (RT-016) study of efaproxiral administered concurrent with whole brain radiation therapy (WBRT) in women with brain metastases from breast cancer. Int J Radiat Oncol Biol Phys 72(1 Suppl): Abstract 110
Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, Jimenez M, Le Rhun E, Pierga JY, Goncalves A et al (2013) Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 14:64–71
Chargari C, Kirova YM, Dieras V, Castro Pena P, Campana F, Cottu PH, Pierga J, Fourquet A (2009) Concurrent capecitabine and whole-brain radiotherapy for treatment of brain metastases in breast cancer patients. J Neurooncol 93:379–384
Olson R, Tyldesley S, Carolan H, Parkinson M, Chhanabhai T, McKenzie M (2011) Prospective comparison of the prognostic utility of the Mini Mental State Examination and the Montreal Cognitive Assessment in patients with brain metastases. Support Care Cancer 19:1849–1855
Acknowledgments
The authors thank all of the patients who participated on this study and who shared their quality of life experiences. In addition, we thank Trinity Urban and the DF/HCC Tumor Imaging Metrics Core (www.tumormetrics.org) for their assistance in central radiology review. We acknowledge the following grant support: American Society of Clinical Oncology Career Development Award, Breast Cancer Research Foundation [NL], the Nancy and Randy Berry Junior Faculty Award [NL], and the Dana-Farber/Harvard Cancer Center Grant CA006516, GlaxoSmithKline.
Ethical Standards
The study was conducted in accordance with the International Conference on Harmonization of Good Clinical Practice guidelines and the Declaration of Helsinki.
Conflict of interest
There are no specific conflicts of interest relevant to this study for any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, N.U., Freedman, R.A., Ramakrishna, N. et al. A phase I study of lapatinib with whole brain radiotherapy in patients with Human Epidermal Growth Factor Receptor 2 (HER2)-positive breast cancer brain metastases. Breast Cancer Res Treat 142, 405–414 (2013). https://doi.org/10.1007/s10549-013-2754-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2754-0