Abstract
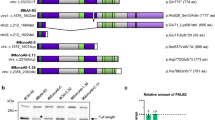
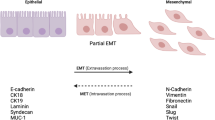
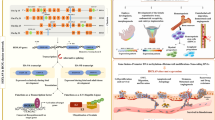
Breast cancer is the second leading cause of cancer death in women in the United States. Metastasis accounts for the death of ~90 % of these patients, yet the mechanisms underlying this event remain poorly defined. WAVE3 belongs to the WASP/WAVE family of actin-binding proteins that play essential roles in regulating cell morphology, actin polymerization, cytoskeleton remodeling, cell motility, and invasion. Accordingly, we demonstrated previously that WAVE3 promotes the acquisition of invasive and metastatic phenotypes by human breast cancers. Herein, we show that transforming growth factor-β (TGF-β) selectively and robustly induced the expression of WAVE3 in metastatic breast cancer cells, but not in their nonmetastatic counterparts. Moreover, the induction of WAVE3 expression in human and mouse triple-negative breast cancer cells (TNBCs) by TGF-β likely reflects its coupling to microRNA expression via a Smad2- and β3 integrin-dependent mechanism. We further demonstrate the requirement for WAVE3 expression in mediating the initiation of epithelial–mesenchymal transition (EMT) programs stimulated by TGF-β. Indeed, stable depletion of WAVE3 expression in human TNBC cells prevented TGF-β from inducing EMT programs and from stimulating the proliferation, migration, and the formation of lamellipodia in metastatic TNBC cells. Lastly, we observed WAVE3 deficiency to abrogate the outgrowth of TNBC cell organoids in 3-dimensional organotypic cultures as well as to decrease the growth and metastasis of 4T1 tumors produced in syngeneic Balb/C mice. Indeed, WAVE3 deficiency significantly reduced the presence of sarcomatoid morphologies indicative of EMT phenotypes in pulmonary TNBC tumors as compared to those detected in their parental counterparts. Collectively, these findings indicate the necessity for WAVE3 expression and activity during EMT programs stimulated by TGF-β; they also suggest that measures capable of inactivating WAVE3 may play a role in alleviating metastasis stimulated by TGF-β.






Similar content being viewed by others
Abbreviations
- 3D:
-
3-Dimensional
- EMT:
-
Epithelial–mesenchymal transition
- ER-α:
-
Estrogen receptor-α
- IHC:
-
Immunohistochemistry
- MEC:
-
Mammary epithelial cell
- PR:
-
Progesterone receptor
- TGF-β:
-
Transforming growth factor-β
- TNBC:
-
Triple-negative breast cancer
References
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752
Carey L, Winer E, Viale G, Cameron D, Gianni L (2010) Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol 7(12):683–692
Carey LA (2010) Directed therapy of subtypes of triple-negative breast cancer. Oncologist 15(Suppl 5):49–56
Siegel R, Naishadham D, Jemal CA (2012) Cancer statistics. CA Cancer J Clin 62(1):10–29
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147(2):275–292
Taylor MA, Parvani JG, Schiemann WP (2010) The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-β in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia 15(2):169–190
Sossey-Alaoui K (2013) Surfing the big WAVE: insights into the role of WAVE3 as a driving force in cancer progression and metastasis. Semin Cell Dev Biol 24:287–297
Sossey-Alaoui K, Downs-Kelly E, Das M, Izem L, Tubbs R, Plow EF (2011) WAVE3, an actin remodeling protein, is regulated by the metastasis suppressor microRNA, miR-31, during the invasion-metastasis cascade. Int J Cancer 129(6):1331–1343
Sossey-Alaoui K, Li X, Cowell JK (2007) c-Abl-mediated phosphorylation of WAVE3 is required for lamellipodia formation and cell migration. J Biol Chem 282(36):26257–26265
Sossey-Alaoui K, Li X, Ranalli TA, Cowell JK (2005) WAVE3-mediated cell migration and lamellipodia formation are regulated downstream of phosphatidylinositol 3-kinase. J Biol Chem 280(23):21748–21755
Sossey-Alaoui K, Ranalli TA, Li X, Bakin AV, Cowell JK (2005) WAVE3 promotes cell motility and invasion through the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp Cell Res 308(1):135–145
Sossey-Alaoui K, Safina A, Li X, Vaughan MM, Hicks DG, Bakin AV, Cowell JK (2007) Down-regulation of WAVE3, a metastasis promoter gene, inhibits invasion and metastasis of breast cancer cells. Am J Pathol 170(6):2112–2121
Takenawa T, Miki H (2001) WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci 114(Pt 10):1801–1809
Kulkarni S, Augoff K, Rivera L, McCue B, Khoury T, Groman A, Zhang L, Tian L, Sossey-Alaoui K (2012) Increased expression levels of WAVE3 are associated with the progression and metastasis of triple negative breast cancer. PLoS ONE 7(8):e42895
Parvani JG, Taylor MA, Schiemann WP (2011) Noncanonical TGF-β signaling during mammary tumorigenesis. J Mammary Gland Biol Neoplasia 16(2):127–146
Wendt MK, Smith JA, Schiemann WP (2010) Transforming growth factor-β-induced epithelial-mesenchymal transition facilitates epidermal growth factor-dependent breast cancer progression. Oncogene 29(49):6485–6498
Wendt MK, Smith JA, Schiemann WP (2009) p130Cas is required for mammary tumor growth and TGF-β-mediated metastasis through regulation of Smad2/3 activity. J Biol Chem 284(49):31145–31156
Wendt MK, Cooper AN, Dwinell MB (2008) Epigenetic silencing of CXCL12 increases the metastatic potential of mammary carcinoma cells. Oncogene 27(10):1461–1471
Wendt MK, Taylor MA, Schiemann BJ, Schiemann WP (2011) Down-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol Biol Cell 22(14):2423–2435
Wendt MK, Schiemann WP (2009) Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-β signaling and metastasis. Breast Cancer Res 11(5):R68
Schiemann WP, Blobe GC, Kalume DE, Pandey A, Lodish HF (2002) Context-specific effects of fibulin-5 (DANCE/EVEC) on cell proliferation, motility, and invasion. Fibulin-5 is induced by transforming growth factor-β and affects protein kinase cascades. J Biol Chem 277(30):27367–27377
Galliher AJ, Schiemann WP (2006) β3 integrin and Src facilitate transforming growth factor-β mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res 8(4):R42
Lee GY, Kenny PA, Lee EH, Bissell MJ (2007) Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 4(4):359–365
Taylor MA, Amin JD, Kirschmann DA, Schiemann WP (2011) Lysyl oxidase contributes to mechanotransduction-mediated regulation of transforming growth factor-β signaling in breast cancer cells. Neoplasia 13(5):406–418
Schiemann WP, Pfeifer WM, Levi E, Kadin ME, Lodish HF (1999) A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood 94(8):2854–2861
Taylor MA, Sossey-Alaoui K, Thompson CL, Danielpour D, Schiemann WP (2013) TGF-β upregulates miR-181a expression to promote breast cancer metastasis. J Clin Invest 123(1):150–163
Ghoshal P, Teng Y, Lesoon LA, Cowell JK (2012) HIF1A induces expression of the WASF3 metastasis-associated gene under hypoxic conditions. Int J Cancer 131(6):E905–E915
Fernando HS, Sanders AJ, Kynaston HG, Jiang WG (2010) WAVE3 is associated with invasiveness in prostate cancer cells. Urol Oncol 28(3):320–327
Aslakson CJ, Miller FR (1992) Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res 52(6):1399–1405
Wendt MK, Schiemann BJ, Parvani JG, Lee YH, Kang Y, Schiemann WP (2013) TGF-β stimulates Pyk2 expression as part of an epithelial-mesenchymal transition program required for metastatic outgrowth of breast cancer. Oncogene 32(16):2005–2015
Sossey-Alaoui K, Bialkowska K, Plow EF (2009) The miR200 family of microRNAs regulates WAVE3-dependent cancer cell invasion. J Biol Chem 284(48):33019–33029
Galliher AJ, Schiemann WP (2007) Src phosphorylates Tyr284 in TGF-β type II receptor and regulates TGF-β stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res 67(8):3752–3758
Galliher-Beckley AJ, Schiemann WP (2008) Grb2 binding to Tyr284 in TβR-II is essential for mammary tumor growth and metastasis stimulated by TGF-beta. Carcinogenesis 29(2):244–251
Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL (2001) Integrin β1 signaling is necessary for transforming growth factor-β activation of p38MAPK and epithelial plasticity. J Biol Chem 276(50):46707–46713
Farina AR, Coppa A, Tiberio A, Tacconelli A, Turco A, Colletta G, Gulino A, Mackay AR (1998) Transforming growth factor-β1 enhances the invasiveness of human MDA-MB-231 breast cancer cells by up-regulating urokinase activity. Int J Cancer 75(5):721–730
McEarchern JA, Kobie JJ, Mack V, Wu RS, Meade-Tollin L, Arteaga CL, Dumont N, Besselsen D, Seftor E, Hendrix MJ, Katsanis E, Akporiaye ET (2001) Invasion and metastasis of a mammary tumor involves TGF-β signaling. Int J Cancer 91(1):76–82
Neil JR, Schiemann WP (2008) Altered TAB 1:IκB kinase interaction promotes transforming growth factor β-mediated nuclear factor-κB activation during breast cancer progression. Cancer Res 68(5):1462–1470
Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, Liu ZY, Costes SV, Cho EH, Lockett S, Khanna C, Chambers AF, Green JE (2008) Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res 68(15):6241–6250
Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, Webster JD, Hoover S, Simpson RM, Gauldie J, Green JE (2010) Metastatic growth from dormant cells induced by a Col-I-enriched fibrotic environment. Cancer Res 70(14):5706–5716
Shibue T, Weinberg RA (2009) Integrin β1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci USA 106(25):10290–10295
Allington TM, Galliher-Beckley AJ, Schiemann WP (2009) Activated Abl kinase inhibits oncogenic transforming growth factor-β signaling and tumorigenesis in mammary tumors. FASEB J 23(12):4231–4243
Hoshino Y, Katsuno Y, Ehata S, Miyazono K (2011) Autocrine TGF-β protects breast cancer cells from apoptosis through reduction of BH3-only protein, Bim. J Biochem 149(1):55–65
Neil JR, Tian M, Schiemann WP (2009) X-linked inhibitor of apoptosis protein and its E3 ligase activity promote transforming growth factor-β-mediated nuclear factor-κB activation during breast cancer progression. J Biol Chem 284(32):21209–21217
Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, Graf S, Ha G, Haffari G, Bashashati A, Russell R, McKinney S, Group M, Langerod A, Green A, Provenzano E, Wishart G, Pinder S, Watson P, Markowetz F, Murphy L, Ellis I, Purushotham A, Borresen-Dale AL, Brenton JD, Tavare S, Caldas C, Aparicio S (2012) The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486(7403):346–352
Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, Sledge GW, Carey LA (2008) Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res 14(24):8010–8018
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121(7):2750–2767
Zipfel PA, Bunnell SC, Witherow DS, Gu JJ, Chislock EM, Ring C, Pendergast AM (2006) Role for the Abi/wave protein complex in T cell receptor-mediated proliferation and cytoskeletal remodeling. Curr Biol 16(1):35–46
Wang R, Mercaitis OP, Jia L, Panettieri RA, Tang DD (2012) Raf-1, actin dynamics and Abl in human airway smooth muscle cells. Am J Respir Cell Mol Biol 48(2):172–180
Lahlou H, Muller WJ (2011) β1-integrins signaling and mammary tumor progression in transgenic mouse models: implications for human breast cancer. Breast Cancer Res 13(6):229
Lindeman GJ, Visvader JE (2010) Insights into the cell of origin in breast cancer and breast cancer stem cells. Asia Pac J Clin Oncol 6(2):89–97
Acknowledgments
Members of the Sossey-Alaoui and Schiemann laboratories are thanked for critical comments and reading of the manuscript. The expertise and guidance provided by the Athymic Animal and Xenograft Core, the Imaging Research Core, and the Tissue Procurement, Histology, and IHC core of the Case Comprehensive Cancer Center are also gratefully acknowledged. Research support was provided in part by the National Institutes of Health to W.P.S. (CA129359) and E.F.P. (HL073311 and HL HL096062), and by the Department of Defense to K.S.-A. (BC073783) and to M.A.T. (BC093128). Additional support was provided to W.P.S. and K.S.-A. by pilot funds from the Case Comprehensive Cancer Center (P30 CA043703).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Taylor, M.A., Davuluri, G., Parvani, J.G. et al. Upregulated WAVE3 expression is essential for TGF-β-mediated EMT and metastasis of triple-negative breast cancer cells. Breast Cancer Res Treat 142, 341–353 (2013). https://doi.org/10.1007/s10549-013-2753-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2753-1