Abstract
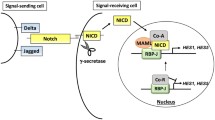
Ayurvedic medicine plants continue to draw attention for the discovery of novel anticancer agents. Withaferin A (WA) is one such small-molecule constituent of the ayurvedic medicine plant Withania somnifera with efficacy against cultured and xenografted human breast cancer cells. However, the mechanism underlying anticancer effect of WA is not fully understood. This study was undertaken to determine the role of Notch signaling in anticancer effects of WA using human breast cancer cells as a model. Notably, Notch signaling is often hyperactive in human breast cancers. Exposure of MDA-MB-231 and MCF-7 human breast cancer cells to pharmacological concentrations of WA resulted in cleavage (activation) of Notch2 as well as Notch4, which was accompanied by transcriptional activation of Notch as evidenced by RBP-Jk, HES-1A/B, and HEY-1 luciferase reporter assays. On the other hand, WA treatment caused a decrease in levels of both transmembrane and cleaved Notch1. The WA-mediated activation of Notch was associated with induction of γ-secretase complex components presenilin1 and/or nicastrin. Inhibition of MDA-MB-231 and MDA-MB-468 cell migration resulting from WA exposure was significantly augmented by knockdown of Notch2 as well as Notch4 protein. Activation of Notch2 was not observed in cells treated with withanone or withanolide A, which are structural analogs of WA. The results of this study indicate that WA treatment activates Notch2 and Notch4, which impede inhibitory effect of WA on breast cancer cell migration.







Similar content being viewed by others
Abbreviations
- WA:
-
Withaferin A
- WE:
-
Withanone
- WLA:
-
Withanolide A
- DMSO:
-
Dimethyl sulfoxide
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- PBS:
-
Phosphate-buffered saline
- siRNA:
-
Small interfering RNA
- shRNA:
-
Small-hairpin RNA
- uPA:
-
Urokinase-type plasminogen activator
- uPAR:
-
uPA receptor
References
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300
Alvarez RH (2010) Present and future evolution of advanced breast cancer therapy. Breast Cancer Res 12(Suppl 2):S1
Higgins MJ, Baselga J (2011) Breast cancer in 2010: novel targets and therapies for a personalized approach. Nat Rev Clin Oncol 8:65–66
Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB (2007) From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol 5:25–37
Gupta SK, Mohanty I, Talwar KK, Dinda A, Joshi S, Bansal P, Saxena A, Arya DS (2004) Cardioprotection from ischemia and reperfusion injury by Withania somnifera: a hemodynamic, biochemical and histopathological assessment. Mol Cell Biochem 260:39–47
Ahmad M, Saleem S, Ahmad AS, Ansari MA, Yousuf S, Hoda MN, Islam F (2005) Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum Exp Toxicol 24:137–147
Owais M, Sharad KS, Shehbaz A, Saleemuddin M (2005) Antibacterial efficacy of Withania somnifera (ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomedicine 12:229–235
Rasool M, Varalakshmi P (2006) Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: an in vivo and in vitro study. Vasc Pharmacol 44:406–410
Devi PU, Sharada AC, Solomon FE (1993) Antitumor and radiosensitizing effects of Withania somnifera (Ashwagandha) on a transplantable mouse tumor, sarcoma-180. Indian J Exp Biol 31:607–611
Widodo N, Kaur K, Shrestha BG, Takagi Y, Ishii T, Wadhwa R, Kaul SC (2007) Selective killing of cancer cells by leaf extract of Ashwagandha: identification of a tumor-inhibitory factor and the first molecular insights to its effect. Clin Cancer Res 13:2298–2306
Padmavathi B, Rath PC, Rao AR, Singh RP (2005) Roots of Withania somnifera inhibit forestomach and skin carcinogenesis in mice. Evid Based Complement Alternat Med 2:99–105
Leyon PV, Kuttan G (2004) Effect of Withania somnifera on B16F–10 melanoma induced metastasis in mice. Phytother Res 18:118–122
Shohat B, Joshua H (1971) Effect of withaferin A on ehrlich ascites tumor cells. II. Target tumor cell destruction in vivo by immune activation. Int J Cancer 8:487–496
Devi PU, Kamath R, Rao BS (2000) Radiosensitization of a mouse melanoma by withaferin A: in vivo studies. Indian J Exp Biol 38:432–437
Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D (2007) Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res 67:246–253
Stan SD, Hahm ER, Warin R, Singh SV (2008) Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res 68:7661–7669
Manoharan S, Panjamurthy K, Menon VP, Balakrishnan S, Alias LM (2009) Protective effect of withaferin-A on tumour formation in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis in hamsters. Indian J Exp Biol 47:16–23
Thaiparambil JT, Bender L, Ganesh T, Kline E, Patel P, Liu Y, Tighiouart M, Vertino PM, Harvey RD, Garcia A, Marcus AI (2011) Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer 129:2744–2755
Stan SD, Zeng Y, Singh SV (2008) Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer 60:51–60
Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV (2011) Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS ONE 6:e23354
Mayola E, Gallerne C, Esposti DD, Martel C, Pervaiz S, Larue L, Debuire B, Lemoine A, Brenner C, Lemaire C (2011) Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis 16:1014–1027
Lee J, Hahm ER, Singh SV (2010) Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis 31:1991–1998
Hahm ER, Lee J, Huang Y, Singh SV (2011) Withaferin A suppresses estrogen receptor-α expression in human breast cancer cells. Mol Carcinog 50:614–624
Kaileh M, Vanden Berghe W, Heyerick A, Horion J, Piette J, Libert C, DeKeukeleire D, Essawi T, Haegeman G (2007) Withaferin A strongly elicits IκB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Biol Chem 282:4253–4264
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90:1371–1388
Leong KG, Karsan A (2006) Recent insights into the role of Notch signaling in tumorigenesis. Blood 107:2223–2233
Mumm JS, Kopan R (2000) Notch signaling: from the outside in. Dev Biol 228:151–165
Hu C, Diévart A, Lupien M, Calvo E, Tremblay G, Jolicoeur P (2006) Overexpression of activated murine Notch1 and Notch3 in transgenic mice blocks mammary gland development and induces mammary tumors. Am J Pathol 168:973–990
Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, Lockwood G, Egan SE (2005) High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res 65:8530–8537
Dickson BC, Mulligan AM, Zhang H, Lockwood G, O’Malley FP, Egan SE, Reedijk M (2007) High-level JAG1 mRNA and protein predict poor outcome in breast cancer. Mod Pathol 20:685–693
Reedijk M, Pinnaduwage D, Dickson BC, Mulligan AM, Zhang H, Bull SB, O’Malley FP, Egan SE, Andrulis IL (2008) JAG1 expression is associated with a basal phenotype and recurrence in lymph node-negative breast cancer. Breast Cancer Res Treat 111:439–448
Koduru S, Kumar R, Srinivasan S, Evers MB, Damodaran C (2010) Notch-1 inhibition by withaferin-A: a therapeutic target against colon carcinogenesis. Mol Cancer Ther 9:202–210
Kim SH, Sehrawat A, Singh SV (2012) Notch2 activation by benzyl isothiocyanate impedes its inhibitory effect on breast cancer cell migration. Breast Cancer Res Treat 134:1067–1079
Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV (2003) Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis 24:891–897
Curry CL, Reed LL, Nickoloff BJ, Miele L, Foreman KE (2006) Notch-independent regulation of Hes-1 expression by c-Jun N-terminal kinase signaling in human endothelial cells. Lab Invest 86:842–852
Kim SH, Sehrawat A, Sakao K, Hahm ER, Singh SV (2011) Notch activation by phenethyl isothiocyanate attenuates its inhibitory effect on prostate cancer cell migration. PLoS ONE 6:e226615
Mödder UI, Oursler MJ, Khosla S, Monroe DG (2011) Wnt10b activates Wnt, Notch, and NFκB pathways in U2OS osteosarcoma cells. J Cell Biochem 112:1392–1402
Wang J, Fu L, Gu F, Ma Y (2011) Notch1 is involved in migration and invasion of human breast cancer cells. Oncol Rep 26:1295–1303
Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, Bundred NJ, Clarke RB (2010) Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res 70:709–718
Lindsay J, Jiao X, Sakamaki T, Casimiro MC, Shirley LA, Tran TH, Ju X, Liu M, Li Z, Wang C, Katiyar S, Rao M, Allen KG, Glazer RI, Ge C, Stanley P, Lisanti MP, Rui H, Pestell RG (2008) ErbB2 induces Notch1 activity and function in breast cancer cells. Clin Transl Sci 1:107–115
Rizzo P, Miao H, D’Souza G, Osipo C, Song LL, Yun J, Zhao H, Mascarenhas J, Wyatt D, Antico G, Hao L, Yao K, Rajan P, Hicks C, Siziopikou K, Selvaggi S, Bashir A, Bhandari D, Marchese A, Lendahl U, Qin JZ, Tonetti DA, Albain K, Nickoloff BJ, Miele L (2008) Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res 68:5226–5235
Rustighi A, Tiberi L, Soldano A, Napoli M, Nuciforo P, Rosato A, Kaplan F, Capobianco A, Pece S, Di Fiore PP, Del Sal G (2009) The prolyl-isomerase Pin1 is Notch1 target that enhances Notch1 activation in cancer. Nat Cell Biol 11:133–142
Schlange T, Matsuda Y, Lienhard S, Huber A, Hynes NE (2007) Autocrine WNT signaling contributes to breast cancer cell proliferation via the canonical WNT pathway and EGFR transactivation. Breast Cancer Res 9:R63
Duffy MJ (2004) The urokinase plasminogen activator system: role in malignancy. Curr Pharm Des 10:39–49
Shimizu M, Cohen B, Goldvasser P, Berman H, Virtanen C, Reedijk M (2011) Plasminogen activator uPA is a direct transcriptional target of the JAG1-Notch receptor signaling pathway in breast cancer. Cancer Res 71:277–286
Annecke K, Schmitt M, Euler U, Zerm M, Paepke D, Paepke S, von Minckwitz G, Thomssen C, Harbeck N (2008) uPA and PAI-1 in breast cancer: review of their clinical utility and current validation in the prospective NNBC-3 trial. Adv Clin Chem 45:31–45
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA142604. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
None of the authors has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J., Sehrawat, A. & Singh, S.V. Withaferin A causes activation of Notch2 and Notch4 in human breast cancer cells. Breast Cancer Res Treat 136, 45–56 (2012). https://doi.org/10.1007/s10549-012-2239-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2239-6