Abstract
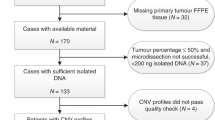
HER2 gene amplification and topoisomerase IIα gene (TOP2A) alteration have been associated with increased benefit from anthracycline compared to non-anthracycline containing adjuvant breast cancer chemotherapy in some but not other studies. Chromosome 17 centromere (CEP17) duplication was measured on TMAs from formalin-fixed paraffin-embedded specimens obtained from 639 of 716 premenopausal women with node positive breast cancer who received cyclophosphamide, epirubicin and fluorouracil (CEF) or cyclophosphamide, methotrexate and fluorouracil (CMF) in the randomized controlled mammary 5 (MA.5) adjuvant trial. The prognostic impact of CEP17 duplication and its interactions with treatment were studied for relapse-free survival (RFS) and overall survival (OS). Overall, CEP17 duplication was not significantly associated with RFS or OS in multivariate analysis. For patients whose tumours had normal CEP17 copy number there were no apparent benefits for CEF compared to CMF for RFS (HR 0.98; 95% CI 0.68–1.42) or OS (HR 1.10; 95% CI 0.72–1.69). For patients whose tumours had CEP17 duplication, there was significant benefit for CEF compared to CMF for RFS (HR 0.54; CI 0.33–0.89) and a trend towards significance for OS (HR 0.64; CI 0.37–1.09). The adjusted P values for interaction between treatment and CEP17 duplication were 0.09 for RFS and 0.13 for OS. This study suggests that CEP17 duplication has a borderline association with clinical responsiveness to anthracycline containing chemotherapy similar to previous results seen with HER2 amplification and TOP2A alteration in MA.5. An appropriately powered meta-analysis is required to discriminate the predictive value of these three candidate markers.


Similar content being viewed by others
References
Di Leo A, Gancberg D, Larsimont D et al (2002) HER-2 amplification and topoisomerase II alpha gene aberrations as predictive markers in node positive breast cancer patients randomly treated either with an anthracycline-based therapy or with cyclophosphamide, methotrexate, and 5-fluorouracil. Clin Cancer Res 8:1107–1116
Di Leo A, Larsimont D, Beauduim M (2001) Her-2 and topoisomerase II alpha as predictive markers in a population of node-positive breast cancer patients randomly treated with adjuvant CMF or epirubicin plus cyclophosphamide. Ann Oncol 12:1081–1089
Pritchard KI, Shepherd LE, O’Malley FA et al (2006) HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med 354:2103–2111
Muss HB, Thor AD, Berry DA et al (1994) C-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. New Engl J Med 330:1260–1266
Paik S, Bryant J, Tan-Chiu E et al (2000) HER2 and choice of adjuvant chemotherapy for invasive breast cancer: National surgical adjuvant breast and bowel project protocol B-15. J Natl Cancer Inst 92:1991–1998
Paik S, Bryant J, Park C et al (1998) ErbB-2 and response to doxorubicin in patients with axillary lymph node positive, hormone receptor negative breast cancer. J Natl Cancer Inst 90:1361–1370
Gennari A, Sormani MP, Pronzato P, Puntoni M, Colozza M, Pfeffer U, Bruzzi P (2008) HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: a pooled analysis of randomized trials. J Natl Cancer Inst 100:14–20
Dhesy-Thind B, Pritchard KI, Messersmith H et al (2008) Her-2/neu in systemic therapy for women with breast cancer: a systemic review. Breast Cancer Res Treat 109:209–229
De Laurentiis M, Arpino G, Massarelli E et al (2005) A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res 11:4741–4748
Slamon DJ, Mackey J, Crown J et al (2007) Role of anthracycline-based therapy in the adjuvant treatment of breast cancer: efficacy analyses determined by molecular subtypes of the disease [abstract]. Breast Cancer Res Treat 106:112 (Abstract 13)
Knoop AS, Knudsen H, Balslev E et al (2005) Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol 23:7483–7490
O’Malley F, Chia S, Tu D et al (2009) Topoisomerase II alpha and responsiveness of breast cancer to adjuvant chemotherapy. J Natl Cancer Inst 101:644–650
Pritchard KI, Messersmith H, Elavathil L et al (2008) Her-2 and topoisomerase II as predictors of response to chemotherapy. J Clin Oncol 26:1–9
Bartlett JMS, Munro A, Cameron DA et al (2008) Type I receptor tyrosine kinase profiles identify patients with enhanced benefit from anthracyclines in the BR9601 adjuvant breast cancer chemotherapy trial. J Clin Oncol 26:5027–5035
Bartlett JMS, Munro A, Dunn JA et al (2010) Predictive markers of anthracycline benefit: a prospectively planned analysis of the UK National Epirubicin Adjuvant Trial (NEAT/BR9601). Lancet Oncol 11:266–274
Di Leo A, Desmedt C, Bartlett JMS et al (2010) Final results of a meta analysis testing HER2 and topoisomerase II genes as predictors of incremental benefit from anthracyclines in breast cancer [abstract]. J Clin Oncol 28:72S
Di Leo A, Isola J, Piette F et al (2008) A meta-analysis of phase III trials evaluating the predictive value of HER2 and topoisomerase II alpha in early breast cancer patients treated with CMF or anthracycline-based adjuvant therapy [abstract]. Breast Cancer Res Treat 107:24s (Abstract 705)
Jarvinen TA, Tanner M, Rantanen V et al (2000) Amplification and deletion of topoisomerase II alpha associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am J Pathol 156:839–847
O’Malley FA, Chia S, Tu D et al (2011) Topoisomerase II alpha protein and responsiveness of breast cancer to adjuvant chemotherapy with CEF compared to CMF in the NCIC CTG randomized MA.5 adjuvant trial. Breast Cancer Res Treat 128:1511–1515
Jarvinen TA, Kononen J, Peltohuikko M, Isola J (1996) Expression of topoisomerase II alpha is associated with rapid cell proliferation, aneuploidy, and c-erbB2 overexpression in breast cancer. Am J Pathol 148:2073–2082
Bartlett JMS, Ellis IO, Dowsett M et al (2007) Human epidermal growth factor receptor 2 status correlates with lymph node involvement in patients with estrogen receptor negative, but with grade in those with ER positive early stage breast cancer suitable for cytotoxic chemotherapy. J Clin Oncol 25:4423–4430
Levine MN, Bramwell VH, Pritchard KI et al (1998) A randomized trial of cyclophosphamide, epirubicin, fluorouracil chemotherapy compared with cyclophosphamide, methrotrexate, fluorouracil in premenopausal women with node positive breast cancer. J Clin Oncol 16:2651–2658
Levine MN, Pritchard KI, Bramwell VH et al (2005) A randomized trial comparing CEF to CMF in premenopausal women with node positive breast cancer: update of NCIC CTG MA.5. J Clin Oncol 23:5166–5170
Elston CW (1991) Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Reinholz MM, Jenkins RB, Hillman DW et al (2007) The clinical significance of poly 17 in the HER2 and N98431 intergroup adjuvant trastuzumab trial [abstract]. Breast Cancer Res Treat 107:S11 (Abstract 36)
Yeh I-T, Martin MA, Robetorye RS et al (2009) Clinical validation of an array CGH test for HER2 status in breast cancer reveals that polysomy 17 is a rare event. Mod Pathol 22:1169–1175
Marchio C, Lambros MB, Gugliotta P et al (2009) Does chromosome 17 centromere copy number predict polysomy in breast cancer? A fluorescence in situ hybridization and microarray-based CGH analysis. J Pathol 219:16–24
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pritchard, K.I., Munro, A., O’Malley, F.P. et al. Chromosome 17 centromere (CEP17) duplication as a predictor of anthracycline response: evidence from the NCIC Clinical Trials Group (NCIC CTG) MA.5 Trial. Breast Cancer Res Treat 131, 541–551 (2012). https://doi.org/10.1007/s10549-011-1840-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1840-4