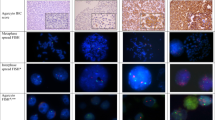
Abstract
Co-amplification of the centromere on chromosome 17 (CEP17) and HER2 can occur in breast cancer. Such aberrant patterns (clusters) on CEP17 can be misleading to calculate the HER2/CEP17 ratio, and thus underreporting of HER2 amplification. We identified 14 breast cancers retrospectively with HER2/CEP17 co-amplification and performed FISH (fluorescence in situ hybridization) with additional chromosome 17 probes (17p11.1–q11.1, 17p11.2–p12, TP53 on 17p13.1, RARA on 17q21.1–3 and TOP2 on 17q21.3–22) to characterize the spanning of the amplicon in these cases. Furthermore, the HER2 status was analyzed by means of HER2 silver in situ hybridization (SISH) and immunohistochemistry (IHC). The co-amplification of HER2/CEP17 was compared between the three institutions. TP53 was eusomic in all cases, 17p11.2–p12 in 79% (11/14), whereas 17p11.1–q11.1 showed chromosomal gain in all cases. RARA was amplified in 10/14 cases (71%) and TOP2 in 3/14 cases (21%). HER2 was amplified with FISH/SISH in all 14 cases. 9/14 tumors were 3+ IHC positive (64%) and 3 cases were 2+ IHC positive. In our cohort the CEP17 amplicon almost always involves the HER2 but not the TOP2 locus. Overall agreement on HER2/CEP17 ratio (when applying ASCO/CAP guidelines) was only 64% (9/14 cases) between the institutions. Discrepant ratios varied from 1.1 to 14.3. The HER2/CEP17 co-amplification is not defined in the ASCO/CAP guidelines, and may result in inaccurate HER2-FISH/SISH status, particularly if only the calculated HER2/CEP17 ratio is reported. It is recommended to report separate CEP17 and HER2 signals in complex HER2/CEP17 patterns.




Similar content being viewed by others
References
Arriola E, Marchio C, Tan DS, Drury SC, Lambros MB, Natrajan R, Rodriguez-Pinilla SM, Mackay A, Tamber N, Fenwick K, Jones C, Dowsett M, Ashworth A, Reis-Filho JS (2008) Genomic analysis of the HER2/TOP2A amplicon in breast cancer and breast cancer cell lines. Lab Investig 88:491–503. doi:10.1038/labinvest.2008.19
Asleson AD, Morgan V, Smith S, Velagaleti GV (2010) Amplification of the RARA gene in acute myeloid leukemia: significant finding or coincidental observation? Cancer Genet Cytogenet 202:33–37. doi:10.1016/j.cancergencyto.2010.06.003
Bartlett JM, Campbell FM, Mallon EA (2008) Determination of HER2 amplification by in situ hybridization: when should chromosome 17 also be determined? Am J Clin Pathol 130:920–926. doi:10.1309/AJCPSDG53BEANCYE
KV Cotran RS, Robbins SL (1994) Robbins pathologic basis of disease. W.B. Saunders Company, Philadelphia
Dellas A, Torhorst J, Schultheiss E, Mihatsch MJ, Moch H (2002) DNA sequence losses on chromosomes 11p and 18q are associated with clinical outcome in lymph node-negative ductal breast cancer. Clin Cancer Res 8:1210–1216
Dietel M, Ellis IO, Hofler H, Kreipe H, Moch H, Dankof A, Kolble K, Kristiansen G (2007) Comparison of automated silver enhanced in situ hybridisation (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists. Virchows Arch 451:19–25. doi:10.1007/s00428-007-0424-5
Fritzsche FR, Bode PK, Moch H, Kristiansen G, Varga Z, Bode B (2010) Determination of the Her-2/neu gene amplification status in cytologic breast cancer specimens using automated silver-enhanced in situ hybridization (SISH). Am J Surg Pathol 34:1180–1185. doi:10.1097/PAS.0b013e3181
Glynn RW, Miller N, Kerin MJ (2010) 17q12–21—the pursuit of targeted therapy in breast cancer. Cancer Treat Rev 36:224–229. doi:10.1016/j.ctrv.2009.12.007
Hicks DG, Yoder BJ, Pettay J, Swain E, Tarr S, Hartke M, Skacel M, Crowe JP, Budd GT, Tubbs RR (2005) The incidence of topoisomerase II-alpha genomic alterations in adenocarcinoma of the breast and their relationship to human epidermal growth factor receptor-2 gene amplification: a fluorescence in situ hybridization study. Hum Pathol 36:348–356. doi:10.1016/j.humpath.2005.01.016
Krishnamurti U, Zarineh A, Atem FD, Silverman JF (2011) Correlation of immunohistochemical expression of p53 with unamplified chromosome 17 polysomy in invasive breast carcinoma. Appl Immunohistochem Mol Morphol 19:28–32. doi:10.1097/PAI.0b013e3181e9bb6f
Lebeau A, Deimling D, Kaltz C, Sendelhofert A, Iff A, Luthardt B, Untch M, Lohrs U (2001) Her-2/neu analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridization. J Clin Oncol 19:354–363
Lebeau A, Turzynski A, Braun S, Behrhof W, Fleige B, Schmitt WD, Grob TJ, Burkhardt L, Holzel D, Jackisch C, Thomssen C, Muller V, Untch M (2010) Reliability of human epidermal growth factor receptor 2 immunohistochemistry in breast core needle biopsies. J Clin Oncol 28:3264–3270. doi:10.1200/JCO.2009.25.9366
Ma Y, Lespagnard L, Durbecq V, Paesmans M, Desmedt C, Gomez-Galdon M, Veys I, Cardoso F, Sotiriou C, Di Leo A, Piccart MJ, Larsimont D (2005) Polysomy 17 in HER-2/neu status elaboration in breast cancer: effect on daily practice. Clin Cancer Res 11:4393–4399. doi:10.1158/1078-0432.CCR-04-2256
Marchio C, Lambros MB, Gugliotta P, Di Cantogno LV, Botta C, Pasini B, Tan DS, Mackay A, Fenwick K, Tamber N, Bussolati G, Ashworth A, Reis-Filho JS, Sapino A (2009) Does chromosome 17 centromere copy number predict polysomy in breast cancer? A fluorescence in situ hybridization and microarray-based CGH analysis. J Pathol 219:16–24. doi:10.1002/path.2574
Nielsen KV, Muller S, Moller S, Schonau A, Balslev E, Knoop AS, Ejlertsen B (2010) Aberrations of ERBB2 and TOP2A genes in breast cancer. Mol Oncol 4:161–168. doi:10.1016/j.molonc.2009.11.001
Ohlschlegel C, Zahel K, Kradolfer D, Hell M, Jochum W (2010) Intratumoral heterogeneity of HER2 status in breast carcinoma. Pathologe 31(Suppl 2):292–295. doi:10.1007/s00292-010-1316-z
Press MF (2006) How is Her-2/neu status established when Her-2/neu and chromosome 17 centromere are both amplified? Am J Clin Pathol 126:673–674. doi:10.1309/GM16-C018-06EF-URX7
Press MF, Sauter G, Buyse M, Bernstein L, Guzman R, Santiago A, Villalobos IE, Eiermann W, Pienkowski T, Martin M, Robert N, Crown J, Bee V, Taupin H, Flom KJ, Tabah-Fisch I, Pauletti G, Lindsay MA, Riva A, Slamon DJ (2010) Alteration of topoisomerase II-alpha gene in human breast cancer: Association with responsiveness to anthracycline-based chemotherapy. J Clin Oncol. doi:10.1200/JCO.2009.27.5644
Reddy KS (2007) Double minutes (dmin) and homogeneously staining regions (hsr) in myeloid disorders: a new case suggesting that dmin form hsr in vivo. Cytogenet Genome Res 119:53–59. doi:10.1159/000109619
Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF (2009) Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol 27:1323–1333. doi:10.1200/JCO.2007.14.8197
Simon R, Nocito A, Hubscher T, Bucher C, Torhorst J, Schraml P, Bubendorf L, Mihatsch MM, Moch H, Wilber K, Schotzau A, Kononen J, Sauter G (2001) Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst 93:1141–1146
Tanner M, Gancberg D, Di Leo A, Larsimont D, Rouas G, Piccart MJ, Isola J (2000) Chromogenic in situ hybridization: a practical alternative for fluorescence in situ hybridization to detect HER-2/neu oncogene amplification in archival breast cancer samples. Am J Pathol 157:1467–1472
Troxell ML, Bangs CD, Lawce HJ, Galperin IB, Baiyee D, West RB, Olson SB, Cherry AM (2006) Evaluation of Her-2/neu status in carcinomas with amplified chromosome 17 centromere locus. Am J Clin Pathol 126:709–716. doi:10.1309/9EYM-6VE5-8F2Y-CD9F
Tubbs RR, Pettay JD, Roche PC, Stoler MH, Jenkins RB, Grogan TM (2001) Discrepancies in clinical laboratory testing of eligibility for trastuzumab therapy: apparent immunohistochemical false-positives do not get the message. J Clin Oncol 19:2714–2721
Vance GH, Barry TS, Bloom KJ, Fitzgibbons PL, Hicks DG, Jenkins RB, Persons DL, Tubbs RR, Hammond ME (2009) Genetic heterogeneity in HER2 testing in breast cancer: panel summary and guidelines. Arch Pathol Lab Med 133:611–612
Vanden Bempt I, Drijkoningen M, De Wolf-Peeters C (2007) The complexity of genotypic alterations underlying HER2-positive breast cancer: an explanation for its clinical heterogeneity. Curr Opin Oncol 19:552–557
Viale G (2009) Be precise! The need to consider the mechanisms for CEP17 copy number changes in breast cancer. J Pathol 219:1–2. doi:10.1002/path.2593
Vranic S, Teruya B, Repertinger S, Ulmer P, Hagenkord J, Gatalica Z (2011) Assessment of HER2 gene status in breast carcinomas with polysomy of chromosome 17. Cancer 117:48–53. doi:10.1002/cncr.25580
Wilson JR, Bateman AC, Hanson H, An Q, Evans G, Rahman N, Jones JL, Eccles DM (2010) A novel HER2-positive breast cancer phenotype arising from germline TP53 mutations. J Med Genet 47:771–774. doi:10.1136/jmg.2010.078113
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145. doi:10.1200/JCO.2006.09.2775
Acknowledgments
The authors wish to thank Dr. Günter Seile for providing case Nr. 14 to the study and Dr. Adriana von Teichman for critical proof-reading and thorough rewriting of the manuscript. There is no conflict of interest for any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varga, Z., Tubbs, R.R., Wang, Z. et al. Co-amplification of the HER2 gene and chromosome 17 centromere: a potential diagnostic pitfall in HER2 testing in breast cancer. Breast Cancer Res Treat 132, 925–935 (2012). https://doi.org/10.1007/s10549-011-1642-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1642-8