Abstract
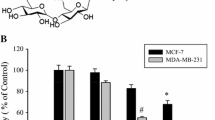
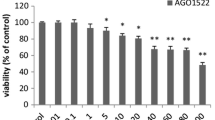
We previously isolated cacalol as a free radical-scavenging compound from Cacalia delphiniifolia which is a traditional Asian herbal plant and is believed to have medicinal effects on cancer. In this report, we demonstrated that cacalol has strong anti-proliferation effect on breast cancer cells and induces apoptosis by activating a pro-apoptotic pathway. We also found that a combination of cacalol and other chemotherapeutic drugs (Taxol and cyclophosphamide) synergistically induced apoptosis and partially overcame chemo-resistance. To further gain a mechanistic insight, we tested a potential inhibitory effect of cacalol on fatty acid synthase gene (FAS) in breast cancer cells, and found that cacalol significantly modulated the expression of the FAS gene, which resulted in apoptosis through activation of DAPK2 and caspase 3. We have also shown that cacalol significantly suppressed the Akt-sterol regulatory element-binding proteins (SREBP) signaling pathway and concomitant transcriptional activation of FAS. In a xenograft model of nude mouse, when cacalol was administered intraperitoneally, tumor growth was significantly suppressed. Importantly, oral administration of cacalol before implanting tumors showed significant preventive effect on tumor growth in the same animal model. Furthermore, the treatment of mice with a combination of low dose of Taxol and cacalol significantly suppressed the tumor growth. Taken together, our results indicate that cacalol induces apoptosis in breast cancer cells and impairs mammary tumor growth in vivo by blocking the expression of the FAS gene through modulation of Akt-SREBP pathway, suggesting that cacalol has potential utility as a chemopreventive and chemotherapeutic agent for breast cancer.





Similar content being viewed by others
Abbreviations
- FAS:
-
Fatty acid synthase.
- ROS:
-
Reactive oxygen species.
- SREBP:
-
Sterol regulatory element-binding protein
- CPA:
-
Cyclophosphamide
- HIF1:
-
Hypoxia-inducible factor-1
References
Kuhajda FP, Jenner K, Wood FD et al (1994) Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci USA 91:6379–6383
Kuhajda FP (2000) Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition 16:202–208
Menendez JA, Lupu R (2004) Fatty acid synthase-catalyzed de novo fatty acid biosynthesis: from anabolic-energy-storage pathway in normal tissues to jack-of-all-trades in cancer cells. Arch Immunol Ther Exp (Warsz) 52:414–426
Kuhajda FP, Katumuluwa AI, Pasternack GR (1989) Expression of haptoglobin related protein and its potential as a tumor antigen. Proc Natl Acad Sci USA 86:1188–1192
Kuhajda FP, Piantadosi S, Pasternack GP (1989) Haptoglobin-related protein (Hpr) epitopes in breast cancer as a predictor of recurrence of the disease. N Engl J Med 321:636–641
Van de Sande T, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV (2002) Role of the phosphatidylinositol 3′-kinase/PTEN/Akt kinase pathway in the overexpression of fatty acid synthase in LNCaP prostate cancer cells. Cancer Res 62:642–646
Yang YA, Han WF, Morin PJ, Chrest FJ, Pizer ES (2002) Activation of fatty acid synthesis during neoplastic transformation: role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Exp Cell Res 279:80–90
Menendez JA, Mehmi I, Atlas E, Colomer R, Lupu R (2004) Novel signaling molecules implicated in tumor-associated fatty acid synthase-dependent breast cancer cell proliferation and survival: role of exogenous dietary fatty acid, p53–21WAF1/CIP1, ERK1/2 MAPK, p27KIP1, BRCA1, and NF-kappaB. Int J Oncol 24:591–608
Porstmann T, Griffiths B, Chung YL et al (2005) PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene 24:6465–6481
Shimano H, Yahagi N, Amemiya-Kudo M et al (1999) Sterol regulatory element binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem 274:35829–35832
Wang D, Sul HS (1998) Insulin stimulation of the fatty acid synthase promoter is mediated by the phosphatidylinositol 3-kinase pathway: involvement of protein kinase B/Akt. J Biol Chem 273:25420–25426
Fleischmann M, Iynedjian PB (2000) Regulation of sterol regulatory-element binding protein 1 gene expression in liver: role of insulin and protein kinase B/cAkt. Biochem J 349:13–17
Kotzka J, Muller-Wieland D, Roth G et al (2000) Sterol regulatory element binding proteins (SREBP)-1a and SREBP-2 are linked to the MAP-kinase cascade. J Lipid Res 41:99–108
Baron A, Migita T, Tang D, Loda M (2004) Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem 91:47–53
Furuta E, Pai S, Zhan R et al (2008) Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res 68:1003–1011
Bandyopadhyay S, Zhan R, Wang Y et al (2006) Mechanism of apoptosis induced by the inhibition of fatty acid synthase in breast cancer cells. Cancer Res 66:5934–5940
Shindo K, Kimura M, Iga M (2004) Potent antioxidative activity of Cacalol, a sesquiterpene contained in Cacalia delphiniifolia Sleb et Zucc. Biosci Biotechnol Biochem 68:1393–1394
Kedrowski B, Hoppe R (2008) A concise synthesis of (±)-cacalol. J Org Chem 73:5177–5179
Fernandes-Alnemri T, Litwack G, Alnemri ES (1994) CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem 269:30761–30764
Naya K, Miyoshi Y, Mori H (1976) The sesquiterpenes of Cacalia species. Chem Lett 73–76
Torihata A, Hanai R, Gong X (2007) Chemical and genetic differentiation of Ligularia tsang-chanensis in Yunnan and Sichuan provinces of China. Chem Biodivers 4:500–507
Inman W, Luo J, Jolad DS, King SR, Cooper R (1999) Antihyperglycemic sesquiterpenes from Psacalium decompositum. J Nat Prod 62:1088–1092
Jimenez-Estrada M, Chilpa RR, Apan TR (2006) Anti-inflammatory activity of cacalol and cacalone sesquiterpenes isolated from Psacalium decompositum. J Ethnopharmacol 105:34–38
Medes G, Thomas A, Weinhouse S (1953) Metabolism of neoplastic tissue IV: a study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res 13:27–29
Swinnen J, Brusselmans K, Verhoeven G (2006) Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care 9:358–365
Hockel M, Vaupel P (2001) Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 93:266–276
Knowles LM, Axelrod F, Browne CD, Smith JW (2004) A fatty acid synthase blockade induces tumor cell-cycle arrest by down-regulating Skp2. J Biol Chem 279:30540–30545
Pizer ES, Jackisch C, Wood FD, Pasternack GR, Davidson NE, Kuhajda FP (1996) Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res 56:2745–2747
Thupari JN, Pinn ML, Kuhajda FP (2001) Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. Biochem Biophys Res Commun 285:217–223
Unruh A, Ressel A, Mohamed HG et al (2003) The hypoxia-inducible factor-1a is a negative factor for tumor therapy. Oncogene 22:3213–3220
Erler JT, Cawthorne CJ, Williams KJ et al (2004) Hypoxia-mediated downregulation of bid and bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol Cell Biol 24:2875–2889
Brown LM, Cowen RL, Debray C et al (2006) Reversing hypoxic cell chemoresistance in vitro using genetic and small molecule approaches targeting hypoxia inducible factor-1. Mol Pharmacol 69:411–418
Hussein D, Estlin EJ, Dive C, Makin GWJ (2006) Chronic hypoxia promotes hypoxia-inducible factor-1a-dependent resistance to etoposide and vincristine in neuroblastoma cells. Mol Cancer Ther 5:2241–2250
Kim HS, Oh JM, Jin DH, Yang KH, Moon EY (2008) Paclitaxel induces vascular endothelial growth factor expression through reactive oxygen species production. Pharmacology 81:317–324
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, W., Furuta, E., Shindo, K. et al. Cacalol, a natural sesquiterpene, induces apoptosis in breast cancer cells by modulating Akt-SREBP-FAS signaling pathway. Breast Cancer Res Treat 128, 57–68 (2011). https://doi.org/10.1007/s10549-010-1076-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1076-8