Abstract
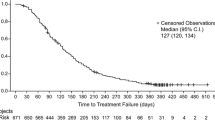
The purpose of this study was to determine the safety and maximum tolerated dose (MTD) of BZL101 (FDA IND# 59,521), an orally delivered aqueous extract from the herb Scutellaria barbata, in women with metastatic breast cancer (MBC). The trial was an open-label, phase 1B, multicenter, dose escalation study. Eligible patients had histologically confirmed breast cancer and measurable stage IV disease. The standard phase 1 “3 + 3” study design was used to determine the MTD. Primary endpoints were toxicity and MTD of BZL101. Secondary outcomes included efficacy based on RECIST criteria. A total of 27 women with a median of 2 prior chemotherapy treatments for metastatic disease were treated in four different dose cohorts. Grade 3 and 4 adverse events (AEs) were uncommon. Dose-limiting toxicities included the following: grade 4 AST elevation, grade 3 diarrhea, grade 3 fatigue, and grade 3 rib pain. Fourteen patients were evaluable according to Response Evaluation Criteria in Solid Tumors. Investigator assessment classified three patients with stable disease for >120 days (21%). One patient was on BZL101 for 449 days and remains stable for 700 + days. Independent radiology review identified three patients with objective tumor regression (>0% and <30%). The MTD was not reached, thus per protocol, the MTD was defined as the maximum administered dose of BZL101 40 g/day. In conclusion, oral administration of BZL101 was safe, well tolerated, and showed promising clinical evidence of anticancer activity in this heavily pretreated population of women with MBC.
Similar content being viewed by others
References
Horner MJ, Krapcho M, Neyman N et al (eds) (2009) SEER Cancer Statistics Review: 1975–2006. Bethesda, MD, National Cancer Institute, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site
Guarneri V, Conte P (2009) Metastatic breast cancer: therapeutic options according to molecular subtypes and prior adjuvant therapy. Oncologist 14(7):645–656
Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792
Campbell MJ, Hamilton B, Shoemaker M, Tagliaferri M, Cohen I, Tripathy D (2002) Antiproliferative activity of Chinese medicinal herbs on breast cancer cells in vitro. Anticancer Res 22(6C):3843–3852
Shoemaker M, Hamilton B, Dairkee SH, Cohen I, Campbell MJ (2005) In vitro anticancer activity of twelve Chinese medicinal herbs. Phytother Res 19(7):649–651
Fong S, Shoemaker M, Cadaoas J et al (2008) Molecular mechanisms underlying selective cytotoxic activity of BZL101, an extract of Scutellaria barbata, towards breast cancer cells. Cancer Biol Ther 7(4):577–586
Shi DY, Xie FZ, Zhai C, Stern JS, Liu Y, Liu SL (2009) The role of cellular oxidative stress in regulating glycolysis energy metabolism in hepatoma cells. Mol Cancer 8:32
Rugo H, Shtivelman E, Perez A et al (2007) Phase I trial and antitumor effects of BZL101 for patients with advanced breast cancer. Breast Cancer Res Treat 105(1):17–28
Le Tourneau C, Lee JJ, Siu LL (2009) Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 101(10):708–720
U.S. Food and Drug Administration: What is a serious adverse event? 8/2009 update. http://www.fda.gov/Safety/MedWatch/HowToReport/ucm053087.htm
Geyer CE, Forster J, Lindquist D et al (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355(26):2733–2743
Butler MS (2004) The role of natural product chemistry in drug discovery. J Nat Prod 67(12):2141–2153
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70(3):461–477
Newman DJ, Cragg GM, Snader KM (2003) Natural products as sources of new drugs over the period 1981–2002. J Nat Prod 66(7):1022–1037
Acknowledgments
This work was supported and funded by Bionovo, Inc. located in Emeryville, CA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perez, A.T., Arun, B., Tripathy, D. et al. A phase 1B dose escalation trial of Scutellaria barbata (BZL101) for patients with metastatic breast cancer. Breast Cancer Res Treat 120, 111–118 (2010). https://doi.org/10.1007/s10549-009-0678-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0678-5