Abstract
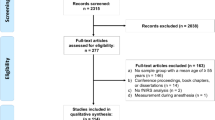
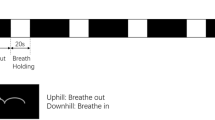
Cerebrovascular reactivity (CVR) is routinely measured as a predictor of stroke in people with a high risk of ischemic attack. Neuroimaging techniques such as emission tomography, magnetic resonance imaging, and transcranial doppler are frequently used to measure CVR even though each technique has its limitations. Functional near-infrared spectroscopy (fNIRS), also based on the principle of neurovascular coupling, is relatively inexpensive, portable, and allows for the quantification of oxy- and deoxy-hemoglobin concentration changes at a high temporal resolution. This study examines the relationship between age and CVR using fNIRS in 45 young healthy adult participants aged 18–41 years (6 females, 26.64 ± 5.49 years) performing a simple breath holding task. Eighteen of the 45 participants were scanned again after a week to evaluate the feasibility of fNIRS in reliably measuring CVR. Results indicate (a) a negative relationship between age and hemodynamic measures of breath holding task in the sensorimotor cortex of 45 individuals and (b) widespread positive coactivation within medial sensorimotor regions and between medial sensorimotor regions with supplementary motor area and prefrontal cortex during breath holding with increasing age. The intraclass correlation coefficient (ICC) indicated only a low to fair/good reliability of the breath hold hemodynamic measures from sensorimotor and prefrontal cortices. However, the average hemodynamic response to breath holding from the two sessions were found to be temporally and spatially in correspondence. Future improvements in the sensitivity and reliability of fNIRS metrics could facilitate fNIRS-based assessment of cerebrovascular function as a potential clinical tool.





Similar content being viewed by others
Data Availability
Data will be available upon request to the corresponding author.
References
Abbott DF, Opdam HI, Briellmann RS, Jackson GD (2005) Brief breath holding may confound functional magnetic resonance imaging studies. Hum Brain Mapp 24:284–290. https://doi.org/10.1002/hbm.20086
Alderliesten T, De Vis JB, Lemmers PM, van Bel F, Benders MJ, Hendrikse J, Petersen ET (2014) Simultaneous quantitative assessment of cerebral physiology using respiratory-calibrated MRI and near-infrared spectroscopy in healthy adults. Neuroimage 85(Pt 1):255–263. https://doi.org/10.1016/j.neuroimage.2013.07.015
Amyot F et al (2020) Assessment of cerebrovascular dysfunction after traumatic brain injury with fMRI and fNIRS. Neuroimage Clin 25:102086. https://doi.org/10.1016/j.nicl.2019.102086
Ayaz H, Onaral B, Izzetoglu K, Shewokis PA, McKendrick R, Parasuraman R (2013) Continuous monitoring of brain dynamics with functional near infrared spectroscopy as a tool for neuroergonomic research: empirical examples and a technological development. Front Hum Neurosci 7:871. https://doi.org/10.3389/fnhum.2013.00871
Brigadoi S et al. (2014) Motion artifacts in functional near-infrared spectroscopy: a comparison of motion correction techniques applied to real cognitive data Neuroimage 85 Pt 1:181–191 https://doi.org/https://doi.org/10.1016/j.neuroimage.2013.04.082
Brigadoi S, Cooper RJ (2015) How short is short? Optimum source-detector distance for short-separation channels in functional near-infrared spectroscopy. Neurophotonics 2:025005. https://doi.org/10.1117/1.NPh.2.2.025005
Bright MG, Murphy K (2013) Reliable quantification of BOLD fMRI cerebrovascular reactivity despite poor breath-hold performance. Neuroimage 83:559–568. https://doi.org/10.1016/j.neuroimage.2013.07.007
Brown MM, Wade JP, Bishop CC, Russell RW (1986) Reactivity of the cerebral circulation in patients with carotid occlusion. J Neurol Neurosurg Psychiatry 49:899–904. https://doi.org/10.1136/jnnp.49.8.899
Catchlove SJ, Parrish TB, Chen Y, Macpherson H, Hughes ME, Pipingas A (2018) Regional cerebrovascular reactivity and cognitive performance in healthy aging. J Exp Neurosci 12:1179069518785151. https://doi.org/10.1177/1179069518785151
Cipolla MJ (2009). In: The Cerebral Circulation. Integrated Systems Physiology: From Molecule to Function. San Rafael (CA),
Coverdale NS, Badrov MB, Shoemaker JK (2017) Impact of age on cerebrovascular dilation versus reactivity to hypercapnia. J Cereb Blood Flow Metab 37:344–355. https://doi.org/10.1177/0271678X15626156
Ellis MJ et al (2016) Neuroimaging assessment of cerebrovascular reactivity in concussion: current concepts methodological considerations, and review of the literature. Front Neurol 7:61. https://doi.org/10.3389/fneur.2016.00061
Fabiani M et al (2014) Taking the pulse of aging: mapping pulse pressure and elasticity in cerebral arteries with optical methods. Psychophysiology 51:1072–1088. https://doi.org/10.1111/psyp.12288
Fierstra J et al (2018) Staging hemodynamic failure with blood oxygen-level-dependent functional magnetic resonance imaging cerebrovascular reactivity: a comparison versus gold standard ((15)O-)H2O-positron emission tomography. Stroke 49:621–629. https://doi.org/10.1161/STROKEAHA.117.020010
Gibbs JM, Wise RJ, Leenders KL, Herold S, Frackowiak RS, Jones T (1985) Cerebral haemodynamics in occlusive carotid-artery disease. Lancet 1:933–934. https://doi.org/10.1016/s0140-6736(85)91712-x
Handwerker DA, Gazzaley A, Inglis BA, D’Esposito M (2007) Reducing vascular variability of fMRI data across aging populations using a breathholding task. Hum Brain Mapp 28:846–859. https://doi.org/10.1002/hbm.20307
Harrison RV, Harel N, Panesar J, Mount RJ (2002) Blood capillary distribution correlates with hemodynamic-based functional imaging in cerebral cortex. Cereb Cortex 12:225–233
Holper L, Scholkmann F, Seifritz E (2015) Time-frequency dynamics of the sum of intra- and extracerebral hemodynamic functional connectivity during resting-state and respiratory challenges assessed by multimodal functional near-infrared spectroscopy. Neuroimage 120:481–492. https://doi.org/10.1016/j.neuroimage.2015.07.021
Hoshi Y (2005) Functional near-infrared spectroscopy: potential and limitations in neuroimaging studies. Int Rev Neurobiol 66:237–266. https://doi.org/10.1016/S0074-7742(05)66008-4
Huppert TJ, Diamond SG, Franceschini MA, Boas DA (2009) HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt 48:D280-298. https://doi.org/10.1364/ao.48.00d280
Jennings JR, Heim AF, Kuan DC, Gianaros PJ, Muldoon MF, Manuck SB (2013) Use of total cerebral blood flow as an imaging biomarker of known cardiovascular risks. Stroke 44:2480–2485. https://doi.org/10.1161/STROKEAHA.113.001716
Jordan J, Shannon JR, Diedrich A, Black B, Costa F, Robertson D, Biaggioni I (2000) Interaction of carbon dioxide and sympathetic nervous system activity in the regulation of cerebral perfusion in humans. Hypertension 36:383–388. https://doi.org/10.1161/01.hyp.36.3.383
Joseph L. Fleiss BL, Myunghee Cho Paik (2003) Statistical Methods for Rates and Proportions. Wiley Series in Probability and Statistics, 3 edn. Wiley, California
Kastrup A, Dichgans J, Niemeier M, Schabet M (1998) Changes of cerebrovascular CO2 reactivity during normal aging. Stroke 29:1311–1314. https://doi.org/10.1161/01.str.29.7.1311
Kastrup A, Li TQ, Glover GH, Moseley ME (1999) Cerebral blood flow-related signal changes during breath-holding. AJNR Am J Neuroradiol 20:1233–1238
Kastrup A, Kruger G, Neumann-Haefelin T, Moseley ME (2001) Assessment of cerebrovascular reactivity with functional magnetic resonance imaging: comparison of CO(2) and breath holding. Magn Reson Imaging 19:13–20
Kim HY (2013) Statistical notes for clinical researchers: evaluation of measurement error 1: using intraclass correlation coefficients. Restor Dent Endod 38:98–102. https://doi.org/10.5395/rde.2013.38.2.98
Kohn JC, Lampi MC, Reinhart-King CA (2015) Age-related vascular stiffening: causes and consequences. Front Genet 6:112. https://doi.org/10.3389/fgene.2015.00112
Lavinio A et al (2008) Cerebrovascular reactivity and autonomic drive following traumatic brain injury. Acta Neurochir Suppl 102:3–7. https://doi.org/10.1007/978-3-211-85578-2_1
Leff DR, Orihuela-Espina F, Elwell CE, Athanasiou T, Delpy DT, Darzi AW, Yang GZ (2011) Assessment of the cerebral cortex during motor task behaviours in adults: a systematic review of functional near infrared spectroscopy (fNIRS) studies. Neuroimage 54:2922–2936. https://doi.org/10.1016/j.neuroimage.2010.10.058
Leoni RF, Mazzetto-Betti KC, Silva AC, Dos Santos AC, de Araujo DB, Leite JP, Pontes-Neto OM (2012) Assessing cerebrovascular reactivity in carotid steno-occlusive disease using MRI BOLD and ASL techniques. Radiol Res Pract 2012:268483. https://doi.org/10.1155/2012/268483
Liu P, De Vis JB, Lu H (2019) Cerebrovascular reactivity (CVR) MRI with CO2 challenge: a technical review. Neuroimage 187:104–115. https://doi.org/10.1016/j.neuroimage.2018.03.047
Lu H et al (2011) Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex 21:1426–1434. https://doi.org/10.1093/cercor/bhq224
Markus HS, Harrison MJ (1992) Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke 23:668–673. https://doi.org/10.1161/01.str.23.5.668
Marshall O et al (2014) Impaired cerebrovascular reactivity in multiple sclerosis. JAMA Neurol 71:1275–1281. https://doi.org/10.1001/jamaneurol.2014.1668
Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS (1991) Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 11:684–689. https://doi.org/10.1038/jcbfm.1991.121
Matteis M, Troisi E, Monaldo BC, Caltagirone C, Silvestrini M (1998) Age and sex differences in cerebral hemodynamics: a transcranial Doppler study. Stroke 29:963–967
McKendrick R, Parasuraman R, Murtza R, Formwalt A, Baccus W, Paczynski M, Ayaz H (2016) Into the wild: neuroergonomic differentiation of hand-held and augmented reality wearable displays during outdoor navigation with functional near infrared spectroscopy. Front Hum Neurosci 10:216. https://doi.org/10.3389/fnhum.2016.00216
Miller S, Mitra K (2017) NIRS-based cerebrovascular regulation assessment: exercise and cerebrovascular reactivity. Neurophotonics 4:041503. https://doi.org/10.1117/1.NPh.4.4.041503
Molavi B, Dumont GA (2012) Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiol Meas 33:259–270. https://doi.org/10.1088/0967-3334/33/2/259
Naqvi J, Yap KH, Ahmad G, Ghosh J (2013) Transcranial Doppler ultrasound: a review of the physical principles and major applications in critical care Int J. Vasc Med 2013:629378. https://doi.org/10.1155/2013/629378
Niu H et al (2013) Test-retest reliability of graph metrics in functional brain networks: a resting-state fNIRS study. PLoS ONE 8:e72425. https://doi.org/10.1371/journal.pone.0072425
Ogasawara K et al (2003) Quantitative measurement of regional cerebrovascular reactivity to acetazolamide using 123I-N-isopropyl-p-iodoamphetamine autoradiography with SPECT: validation study using H2 15O with PET. J Nucl Med 44:520–525
Oudegeest-Sander MH, van Beek AH, Abbink K, Olde Rikkert MG, Hopman MT, Claassen JA (2014) Assessment of dynamic cerebral autoregulation and cerebrovascular CO2 reactivity in ageing by measurements of cerebral blood flow and cortical oxygenation. Exp Physiol 99:586–598. https://doi.org/10.1113/expphysiol.2013.076455
Peebles KC, Ball OG, MacRae BA, Horsman HM (1985) Tzeng YC (2012) Sympathetic regulation of the human cerebrovascular response to carbon dioxide. J Appl Physiol 113:700–706. https://doi.org/10.1152/japplphysiol.00614.2012
Peng SL, Chen X, Li Y, Rodrigue KM, Park DC, Lu H (2018) Age-related changes in cerebrovascular reactivity and their relationship to cognition: a four-year longitudinal study. Neuroimage 174:257–262. https://doi.org/10.1016/j.neuroimage.2018.03.033
Phillips AA, Ainslie PN, Krassioukov AV, Warburton DE (2013) Regulation of cerebral blood flow after spinal cord injury. J Neurotrauma 30:1551–1563. https://doi.org/10.1089/neu.2013.2972
Pinti P, Aichelburg C, Gilbert S, Hamilton A, Hirsch J, Burgess P, Tachtsidis I (2018) A review on the use of wearable functional near-infrared spectroscopy in naturalistic environments(). Jpn Psychol Res 60:347–373. https://doi.org/10.1111/jpr.12206
Raz N et al (1997) Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex 7:268–282. https://doi.org/10.1093/cercor/7.3.268
Reinhard M et al (2014) Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology 83:1424–1431. https://doi.org/10.1212/WNL.0000000000000888
Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C (2003) Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci 23:3295–3301
Scarapicchia V, Brown C, Mayo C, Gawryluk JR (2017) Functional magnetic resonance imaging and functional near-infrared spectroscopy: insights from combined recording studies. Front Hum Neurosci 11:419. https://doi.org/10.3389/fnhum.2017.00419
Scholkmann F, Kleiser S, Metz AJ, Zimmermann R, Mata Pavia J, Wolf U, Wolf M (2014) A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 85(Pt 1):6–27. https://doi.org/10.1016/j.neuroimage.2013.05.004
Selb J, Boas DA, Chan ST, Evans KC, Buckley EM, Carp SA (2014) Sensitivity of near-infrared spectroscopy and diffuse correlation spectroscopy to brain hemodynamics: simulations and experimental findings during hypercapnia. Neurophotonics. https://doi.org/10.1117/1.NPh.1.1.015005
Silvestrini M, Troisi E, Matteis M, Cupini LM, Bernardi G (1996a) Effect of smoking on cerebrovascular reactivity. J Cereb Blood Flow Metab 16:746–749. https://doi.org/10.1097/00004647-199607000-00027
Silvestrini M, Troisi E, Matteis M, Cupini LM, Caltagirone C (1996b) Transcranial Doppler assessment of cerebrovascular reactivity in symptomatic and asymptomatic severe carotid stenosis. Stroke 27:1970–1973
Tan CH et al (2017) Mapping cerebral pulse pressure and arterial compliance over the adult lifespan with optical imaging. PLoS ONE 12:e0171305. https://doi.org/10.1371/journal.pone.0171305
Tancredi FB, Hoge RD (2013) Comparison of cerebral vascular reactivity measures obtained using breath-holding and CO2 inhalation. J Cereb Blood Flow Metab 33:1066–1074. https://doi.org/10.1038/jcbfm.2013.48
Terribilli D et al (2011) Age-related gray matter volume changes in the brain during non-elderly adulthood. Neurobiol Aging 32:354–368. https://doi.org/10.1016/j.neurobiolaging.2009.02.008
Tomoto T, Riley J, Turner M, Zhang R, Tarumi T (2019) Cerebral vasomotor reactivity during hypo- and hypercapnia across the adult lifespan. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678X19828327
Villringer A, Chance B (1997) Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci 20:435–442. https://doi.org/10.1016/s0166-2236(97)01132-6
Wang L, Su L, Shen H, Hu D (2012) Decoding lifespan changes of the human brain using resting-state functional connectivity MRI. PLoS ONE 7:e44530. https://doi.org/10.1371/journal.pone.0044530
Wilcox T, Biondi M (2015) fNIRS in the developmental sciences. Wiley Interdiscip Rev Cogn Sci 6:263–283. https://doi.org/10.1002/wcs.1343
Wu K, Taki Y, Sato K, Qi H, Kawashima R, Fukuda H (2013) A longitudinal study of structural brain network changes with normal aging. Front Hum Neurosci 7:113. https://doi.org/10.3389/fnhum.2013.00113
Zuo XN et al (2010) The oscillating brain: complex and reliable. Neuroimage 49:1432–1445. https://doi.org/10.1016/j.neuroimage.2009.09.037
Acknowledgements
This study was funded by a fellowship from NJCSCR (CSCR15FEL002) to KDK and BBB and a Grant from NSF (MRI CBET 1428425) to BBB and TLA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Christoph M. Michel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karunakaran, K.D., Ji, K., Chen, D.Y. et al. Relationship Between Age and Cerebral Hemodynamic Response to Breath Holding: A Functional Near-Infrared Spectroscopy Study. Brain Topogr 34, 154–166 (2021). https://doi.org/10.1007/s10548-021-00818-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-021-00818-4