Abstract
Prosocial behavior is of vital importance for the smooth functioning of society. However, the propensity to behave in a prosocial manner is characterized by vast individual differences. In order to reveal the sources of these differences, some studies have used objective, task-independent neural traits, for instance resting electroencephalography (EEG). Despite providing valuable insights into the neural signatures of several domains of prosociality, each of these studies has only focused on one single domain. Here, we exposed 137 participants to different social dilemma situations in order to obtain a measure of the individuals’ domain-general prosociality and recorded multi-channel task-independent, resting EEG. Using a source-localization technique, we found that resting current density within the temporo-parietal junction in two beta bands (beta2 and beta3) was positively associated with domain-general prosociality. This is the first demonstration of neural signatures underlying individual differences in the propensity to behave in a prosocial manner across different social situations.
Similar content being viewed by others
Introduction
The ability to behave in a prosocial manner is a prerequisite for large-scale social living. However, prosocial behavior is characterized by vast inter-individual heterogeneity (Kurzban and Houser 2001; Andreoni and Miller 2002; Fischbacher and Gächter 2010). Attempts to investigate the origins of this heterogeneity via personality questionnaires have been only moderately successful (e.g., Becker et al. 2012; Epstein et al. 2016). One reason for this might lie in the fact that self-reporting is susceptible to various sources of bias, including socially desirable responding, random responding, and demand characteristics (Edwards 1957; Nichols and Maner 2008). As an alternative, some studies in the fields of social neuroscience and neuroeconomics applied a so-called neural trait approach, which allows for objective and stable measurement of dispositional differences (for a review, see Nash et al. 2015). Neural traits are much like neural ‘fingerprints’ and have been used to reveal sources of individual differences in various aspects of prosociality, such as altruism (Morishima et al. 2012), cooperation (Fermin et al. 2016), costly punishment (Knoch et al. 2010), honesty (Baumgartner et al. 2013), or reciprocity (Watanabe et al. 2014).
Although these studies have provided valuable insights into the neural signatures of several domains of prosociality, each of them has only focused on one single domain. However, as previous behavioral studies have documented the existence of a domain-general prosocial ‘phenotype’ (e.g., Peysakhovich et al. 2014), it is important to investigate the neural signatures underlying prosocial behavior in more than one single domain of prosociality.
Here, we recorded task-independent resting electroencephalogram (EEG) of 137 participants before they were subjected to different social dilemma situations. Social dilemma situations have been extensively used to model complex social interactions and allow for rigorous empirical investigations. Each of the situations used in this study was characterized by a unique incentive structure, and participants were required to allocate money amongst themselves and other participants. In order to measure domain-general prosociality, which is the common denominator of prosocial behavior across the different social situations, we applied a factor analysis.
Task-independent EEG at rest constitutes an ideal neural trait measure due to its high specificity (i.e., the extent to which an EEG pattern is uniquely associated with a given person; Dünki et al. 2000; Näpflin et al. 2007) and high stability over time (e.g., Dünki et al. 2000; Näpflin et al. 2007; Cannon et al. 2012). In addition, it has been used to reveal sources of individual differences in time preferences (Gianotti et al. 2012), risk preferences (Gianotti et al. 2009; Studer et al. 2013), and social preferences (Knoch et al. 2010; Baumgartner et al. 2013; for a review, see; Nash et al. 2015). Hence, resting EEG is a promising tool to investigate possible sources of individual differences in domain-general prosociality.
Given that this was the first study of its kind, we conducted exploratory whole-brain-corrected analyses without any a-priori hypotheses to investigate the neural signatures of individual differences in domain-general prosociality.
Materials and Methods
Participants
We collected data from 137 (105 female) right-handed, German-speaking participants, recruited at the University of Bern. All participants indicated that they had no current or previous history of neurological or psychiatric disorders or alcohol or drug abuse. Four participants were excluded from the analyses due to technical problems during the EEG or behavioral recordings. The mean age of the remaining 133 participants (102 female) was 21 years (SD = 3). The study was approved by the local ethics committee. All participants provided written, informed consent and were informed of their right to discontinue participation at any time. Participants were remunerated with a flat fee of 40 Swiss francs (CHF 1 ≈ USD 1) in addition to the money earned in the social dilemma situations. We recruited participants for 1 academic year and collected as much data as possible during that time. Data was collected in a single wave and then analyzed after the testing was complete. Data are available upon request.
Procedure
The electroencephalographic and behavioral data collections were separated by several weeks. First, participants completed the EEG recording in our EEG laboratory. After providing written informed consent, participants completed the Positive and Negative Affect Schedule (PANAS, Watson et al. 1988) and a handedness inventory (Chapman and Chapman 1987). Participants were seated in a sound-attenuating, electrically shielded chamber with dim illumination and an intercom connection to the experimenters. Participants were instructed that the EEG recording was to be conducted while they rested with their eyes alternately open or closed. The resting EEG protocol consisted of the participants resting for 20 s with their eyes open, followed by 40 s with their eyes closed; this was repeated five times. The instructions regarding eye opening/closing were provided via the intercom. Data analysis was based on the 200 s eyes-closed condition.
Participants were then subjected to four social dilemma situations in our behavioral laboratory with 24 interconnected computer terminals. Moreover, participants filled out the Honesty-Humility scale of the HEXACO questionnaire (Ashton and Lee 2009), which has been associated with individual differences in prosociality (Hilbig et al. 2012; Aghababaei et al. 2014; Thielmann and Hilbig 2014).
EEG Recording and Pre-processing
Resting EEG was continuously recorded using 60 Ag–AgCl electrodes mounted in an elastic cap and placed according to the international 10–10 system (Nuwer et al. 1998). The electrode at position FCz was the recording reference, while the electrode at position CPz served as the ground electrode. Data were recorded at a sampling rate of 500 Hz (bandwidth: 0.1–250 Hz). Horizontal electrooculographic (EOG) signals were recorded at the left and right outer canthi, and vertical EOGs were recorded below the right eye. Impedances were maintained at < 10 kΩ. Eye-movement artifacts were removed using independent component analysis. After an automatic artifact rejection (maximal allowed voltage step: 15 µV; maximal allowed amplitude: ± 100 µV; minimal allowed activity in intervals of 100 ms: 0.5 µV), data were visually inspected to eliminate residual artifacts. The data were then recomputed against the average reference. All artifact-free 2 s epochs were extracted. On average, 87.1 epochs (SD = 16.7) per participant were eventually available. A Fast Fourier Transformation (using a square window) was applied to each epoch and channel to compute the power spectra with 0.5 Hz resolution. The spectra for each channel were averaged over all epochs for each participant. Absolute power values were integrated for the following seven independent frequency bands (Kubicki et al. 1979): delta (1.5–6 Hz), theta (6.5–8 Hz), alpha1 (8.5–10 Hz), alpha2 (10.5–12 Hz), beta1 (12.5–18 Hz), beta2 (18.5–21 Hz), and beta3 (21.5–30 Hz).
Standardized low-resolution electromagnetic tomography (sLORETA; Pascual-Marqui 2002) was used to estimate the intracerebral electrical sources that generated the scalp-recorded activity at each of the EEG frequency bands. The sLORETA method is a properly standardized, discrete, 3D distributed, linear, minimum norm inverse solution that allows for localization of the intracerebral sources of scalp-recorded electromagnetic signals. The particular form of standardization used in sLORETA endows the tomography with the property of exact localization to test point sources, and thereby yields images of standardized current density with exact localization, albeit with low spatial resolution (i.e., neighboring neural sources will be highly correlated). sLORETA has been validated in several simultaneous EEG/fMRI studies (Mobascher et al. 2009a, b) and in an EEG localization study for epilepsy (Rullmann et al. 2009). In the current implementation of sLORETA, computations are conducted in a realistic head model using the MNI152 template (Mazziotta et al. 2001), with the 3D solution space restricted to cortical gray matter, as determined by the probabilistic Talairach atlas (Lancaster et al. 2000). The intracerebral volume is partitioned in 6239 voxels at 5 mm spatial resolution. Thus, sLORETA images represent the standardized electric activity at each voxel in neuroanatomic Montreal Neurological Institute (MNI) space as the exact magnitude of the estimated current density. Using the automatic regularization method in the sLORETA software, we selected the transformation matrix with the signal-to-noise ratio set to ten. To reduce confounds that have no regional specificity, for each participant, sLORETA images were normalized to a total power of one and then log-transformed before statistical analyses. Due to the higher number of female participants, we first regressed the putative sex-influence out of the sLORETA images. The standardized sLORETA residuals were then used for all further analyses.
Measurements of Domain-General Prosociality
A total of nine behavioral sessions were conducted with an average number of 16 participants per session. At the beginning of each session, participants were randomly assigned to cubicles where they made their decisions with complete anonymity from the other participants. They were not allowed to talk to each other. Participants were confronted with four social dilemma situations (see below) that measured two domains of prosociality: cooperation (public goods game; PGG) and generosity (dictator game; DG). Participants played one trial in each of the four social dilemma situations. Control questions ensured participants’ understanding of the social dilemma situations. There was no time constraint when participants made their decisions. Participants received feedback on the other participants’ choices at the end of the experiment.
Situation 1 (PGG-2)
Participants were assigned to a group of four. Each participant was then endowed with 20 monetary units (1 MU = 0.5 CHF, 20 MUs = 10 CHF) and asked to indicate how many MUs they were willing to contribute to a public good (one-shot). Each contributed MU was multiplied by 2 and split equally among the four group members. A participant’s earning consisted of all the MUs earned from the public good as well as the MUs not contributed in the first place. After their contribution decision, we asked participants how many MUs they believed others had contributed on average.
Situation 2 (PGG-1.2)
The group size and initial endowment were the same as in the first social dilemma situation. However, here each MU contributed to the public good (one-shot) was multiplied by 1.2. Thus, if a participant contributed 20 MUs to the public good and the other three players did not contribute at all, the monetary consequences for this participant were worse in this situation (a reduction of 14 MUs) than in Situation 1 (namely a reduction of 10 MUs). Hence, a prosocial contribution decision in Situation 2 indicated a higher level of prosociality than the same contribution decision in Situation 1. As in Situation 1, after their contribution decisions, we asked participants how many MUs they believed others had contributed on average.
Situation 3 (PGG-3.2)
As in Situation 2, group size and initial endowment remained the same. However, here each MU contributed to the public good (one-shot) was multiplied by 3.2. In the example of a participant who contributed 20 MUs to the public good while the other three players did not contribute at all, the monetary consequences for this participant were less severe (namely a reduction of the initial endowment by 4 MUs) than those in both previous situations (Situation 1: reduction of 10 MUs; Situation 2: reduction of 14 MUs). Thus, in this situation, contributing was minimally costly and maximally beneficial to others, while in Situation 2, contributing was maximally costly and minimally beneficial to others. In Situation 1, the costs and benefits were in between those of the latter situations. That is, the same contribution was—by definition—more ‘expensive’ when the multiplier (i.e., the number by which each point contributed to the public good was multiplied, specifically, 1.2, 2, or 3.2) was small. Such variation is likely to have influenced individuals with different levels of prosociality in a different manner. Whereas a highly prosocial participant might have been influenced only a little by the context and therefore also cooperated in an “expensive” context such as Situation 2, a participant with a lower inclination towards prosociality might have been strongly influenced by the context and did not contribute when the multiplier was small, as in Situation 2. This notion was documented for instance by Isaac and Walker (1988), demonstrating that a decrease in the multiplier can lead to a significant increase in free-riding behavior. In a similar fashion to Situations 1 and 2, in Situation 3 we asked participants immediately after making their contribution decisions how many MUs they believed others had contributed on average. Situations 2 and 3 were presented in a counterbalanced order.
Situation 4 (DG)
The participants played the role of a ‘dictator’. They were endowed with 10 MUs (5 CHF) and were asked to decide how many MUs to keep and how many to give to an anonymous recipient (one-shot). The participants were informed in advance that at the end of the study, their allocation was randomly assigned to another participant and implemented accordingly.
Statistical Analyses
To capture the domain-general prosociality, we performed a factor analysis on participants’ prosocial behavior in the different situations. The contributions in the social dilemma situations were z-transformed and subjected to a principal axis factoring procedure. Factors based only on the common variance of the four situations were extracted from the original correlation matrix. The number of factors to be extracted was determined by the criterion wherein the eigenvalues were ≥ 1. The resulting factor scores were obtained using regressions. Since only one factor was extracted, we refer to this factor score as the ‘Domain-general Prosociality Index.’
The primary aim of this study was to examine whether domain-general prosociality can be explained by a task-independent neural trait measure. Thus, we conducted whole-brain voxel-wise Spearman’s rank correlations (separately for each frequency band) using the Domain-general Prosociality Index as the dependent variable. Correction for multiple testing was implemented by means of a nonparametric randomization approach (Nichols and Holmes 2002). The nonparametric randomization approach was used to estimate empirical probability distributions (number of randomizations used: 5000) and the corresponding corrected (for multiple comparisons) critical probability thresholds. For regions that displayed significant, whole-brain corrected correlations, the voxels with the strongest correlations (peak voxels) were then used for the construction of spherical regions of interest (ROIs; radius: 10 mm around the peak voxel). Mean current density within the ROIs was calculated and used for visualization and for additional regression analyses.
A conjunction analysis was carried out as a second approach to examine the neural traits underlying domain-general prosociality. Four whole-brain corrected Spearman’s rank correlation images between resting EEG and contribution decisions in the social dilemma situations were computed separately. A conjunction was then performed to quantify the overlap between the four correlations images.
Results
Behavioral Results
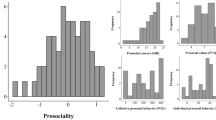
As illustrated in Fig. 1, the four distributions of the participants’ contributions differed significantly between the different social dilemma situations (Wilcoxon signed rank tests: all six comparisons p < 0.001).
Factor analysis revealed an underlying structure composed of one single factor. The factor loadings were 0.92, 0.49, 0.60, and 0.31 for Situations 1–4 (that is, PGG-2, PGG-1.2, PGG-3.2 and DG), respectively. No other factor had an eigenvalue that exceeded 1 (eigenvalue of the next factor: 0.87). This factor, hereby referred to as ‘Domain-general Prosociality Index’, accounted for 49.9% of the variance in the four decisions. We observed large inter-individual differences in domain-general prosociality (Fig. 2). The Domain-general Prosociality Index varied from − 1.78 to 1.88 (M = 0, SD = 0.93). There was no correlation between the Domain-general Prosociality Index and the honesty-humility scale [r(131) = − 0.079, p = 0.37], nor with positive affect [r(131) = − 0.141, p = 0.11] or negative affect [r(131) = − 0.060, p = 0.50].
Brain Results
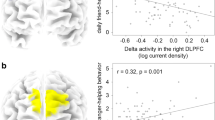
To study the putative neural traits underlying individual differences in domain-general prosociality, we conducted whole-brain Spearman’s rank correlations with the Domain-general Prosociality Index as the dependent variable. Using sLORETA as a source localization technique to estimate the intracerebral sources underlying scalp-recorded resting EEG, we found that the Domain-general Prosociality Index was positively correlated with task-independent resting EEG in the beta2 and beta3 frequency bands. The results in the two beta bands were largely overlapping and comprised a cluster in the right temporo-parietal junction (TPJ, BAs 21, 22, 37, 39, and 40). Specifically, in the beta2 band, all 30 significant voxels fell into one cluster in the right TPJ (BAs 21, 22, 37, 39, and 40; MNI coordinates peak voxel: x = 60, y = − 60, z = 15, Fig. 3a). In the beta3 band, all 19 significant voxels fell into one cluster that also peaked in the right TPJ (BAs 21, 22, 37, 39, and 40; MNI coordinates peak voxel: x = 65, y = − 55, z = 5, Fig. 3b). Spearman’s rank correlations conducted with the two ROIs (spheres of 10 mm radius around the peak voxels) revealed positive correlation coefficients of rs(131) = 0.28, p = 0.001 in beta2 and rs(131) = 0.26, p = 0.003 in beta3.
Relationship between resting beta current density and the Domain-general Prosociality Index in the right temporo-parietal junction. On the left, locations of the voxels that exhibited significant correlations (whole-brain corrected) in the beta 2 (a) and beta3 (b) frequency bands are indicated in red (p < 0.05). On the right, the scatter plots, based on a 10 mm spherical ROI around the corresponding peak voxels, demonstrate the positive association between the resting beta current density (residuals) and the Domain-general Prosociality Index, including regression lines (in red), and confidence intervals (95%)
Controlling for the belief regarding others’ contributions demonstrated that the correlation between resting beta current density in the TPJ and the Domain-general Prosociality Index remained significant: rs(130) = 0.21, p = 0.02 in beta2; rs(130) = 0.19, p = 0.03 in beta3 in the right TPJ.
Our findings were specific to the beta2 and beta3 frequency bands and the right TPJ, as no significant correlations were found in any other EEG frequency band, and in no other brain region was the resting beta2 or beta3 current density correlated with the Domain-general Prosociality Index.
Conjunction analysis confirmed the association between the resting beta current density in the right TPJ and the contributions in each of the four social dilemma situations. Specifically, in the beta2 band (Fig. 4), 23 significant voxels fell into one cluster in the right TPJ (BAs 21, 22, 39, and 40; MNI coordinates peak voxel: x = 60, y = − 55, z = 20). The Spearman’s rank correlations between resting beta2 current density and the contributions in the four social dilemma situations revealed positive significant correlation coefficients of rs(131) = 0.22, p = 0.006 in the PGG-1.2 (MNI coordinates of peak voxel: x = 65, y = − 45, z = 25), rs(131) = 0.27, p < 0.001 in the PGG-2 (MNI coordinates of peak voxel: x = 60, y = − 60, z = 15), rs(131) = 0.21, p = 0.008 in the PGG-3.2 (MNI coordinates of peak voxel: x = 65, y = − 50, z = 20), and rs(131) = 0.30, p < 0.001 in the DG (MNI coordinates of peak voxel: x = 60, y = − 55, z = 20). The four correlation coefficients (between resting beta2 current density in TPJ and PGG-1.2, PGG-2, PGG-3.2, DG) did not differ significantly, as demonstrated by the Meng’s test for comparing correlated non-parametric correlation coefficients (all p > 0.4).
Conjunction analysis. In the center, the locations of all voxels that proved to be significant in the conjunction analyses for the four brain correlation images (whole-brain corrected) in the beta2 band is depicted. Red indicates p < 0.05. The scatter plots, based on a 10 mm spherical ROI around the corresponding peak voxel demonstrate the positive association between the resting beta2 current density (residuals) and the contributions in the different social dilemma situations. Regression lines and confidence intervals (95%) are indicated in red
In the beta3 band, eight significant voxels fell into one cluster in the right TPJ (BAs 21 and 22; MNI coordinates peak voxel: x = 65, y = − 55, z = 5).
Discussion
In this study, we identified a specific neural trait marker—resting fast-wave oscillations originating from the temporo-parietal junction (TPJ)—for domain-general prosociality. More specifically, higher levels of beta2 and beta3 current density in the right TPJ were associated with higher prosocial contributions across different social dilemma situations.
Previous neural trait studies have revealed sources of individual differences for several domains of prosociality in different brain regions, for instance in the right TPJ (e.g., Morishima et al. 2012), dorsolateral prefrontal cortex (Knoch et al. 2010; Fermin et al. 2016), or anterior insula (Baumgartner et al. 2013; Watanabe et al. 2014). However, each of these studies has only focused on one single domain. In contrast to previous studies, we measured domain-general prosociality and investigated the neural signatures underlying an individual’s propensity to behave prosocially across different situations. We propose that resting EEG current density in the TPJ reflects an individual’s propensity to engage in prosocial behavior across situations. As confirmed by the conjunction analysis, prosocial decisions in all four social dilemma situations were indeed associated with higher resting beta current density in the TPJ.
To ensure that we obtained a pure measure of prosociality and to control for possible strategies, we asked our participants after their contribution decisions what they believed others contributed on average. Since the participants’ final pay-off also depended on the other participants’ contribution to the public good, the participants’ contribution decision could have been influenced by their belief of the other participants’ behavior. For example, a participant could strategically decide to contribute half of their endowment because they assumed that the other three players would contribute a similar amount. Another participant could contribute half of their endowment, according to their prosocial inclination, without strategically considering others’ decisions. Clearly, the same contribution of these two individuals does not indicate the same level of domain-general prosociality. To control for this possible confound, we ran additional analyses that accounted for the participants’ estimation of the others’ average contributions. The results of these additional analyses corroborated the finding that resting EEG current density in the right TPJ was significantly associated with pure domain-general prosociality.
One popular account of TPJ function posits that it allows attention to be shifted away from the self to focus on others’ perspectives (e.g., Lamm et al. 2016; for a recent review see; Steinbeis 2016) and to overcome one’s self-centered perspective (Hare et al. 2010; Strombach et al. 2015; Soutschek et al. 2016). As prosociality involves sacrificing resources for the benefit of others, prosocial decisions are likely to require a shift in attention away from the subject’s own state to focus on the needs of others. Interestingly, a lack in the capacity of distinction between the self and the others can indeed have deleterious effects on prosocial behavior (e.g., Decety and Lamm 2009). Furthermore, evidence from a modulation study suggests that the TPJ is causally involved in overcoming self-centeredness, rendering behavior prosocial (Soutschek et al. 2016). This leads to the speculation that people with higher resting beta current density in the TPJ, that is, people with higher abilities in self-other distinction and in overcoming self-centeredness, are more likely to exhibit higher levels of domain-general prosociality than their counterparts with lower abilities. Support for this speculation may also be found in online EEG studies that showed that beta oscillations originating from the TPJ play an important role when attempting to estimate other´s preferences (Park et al. 2018) or when differentiating between an ingroup or outgroup member (Riecansky et al. 2014).
Finally, we found that domain-general prosociality was not related to the honesty-humility scale of the HEXACO questionnaire. This result contrasts with previous studies that demonstrated a positive association between honesty-humility and different forms of prosociality (e.g., Hilbig et al. 2012; Aghababaei et al. 2014; Thielmann and Hilbig 2014). However, in these studies, prosociality was measured either with self-reports or with economic games that were entirely hypothetical and without financial consequences (however, see Zhao et al. 2016).
In sum, we believe that this is the first study to demonstrate that a specific neural trait marker—task-independent resting beta current density in the TPJ—explains individuals’ variation in domain-general prosociality. These results provide neural evidence as to why some people consistently behave more prosocially than others in different situations. The capacity to differentiate between the self and the others and to overcome self-centeredness might be a fundamental prerequisite for domain-general prosociality. A deeper understanding of the neural underpinnings of domain-general prosociality will help improving prosocial behavior, for instance in patients with autism spectrum disorder (a disorder characterized by a hypoactivation in the TPJ; e.g., Lombardo et al. 2011; Pantelis et al. 2015). However, with our results we cannot completely rule out the possibility that resting beta current density in the TPJ is not associated with behaviors that would still fall under the domain of prosociality, like for example, providing instrumental help or comforting.
Although neural traits are highly stable across time, they are not immutable. It has been well documented that repeated practice of skills as well as techniques such as neurofeedback or meditation have the capacity to induce permanent changes in neural structure or function (e.g., Lazar et al. 2005; Takeuchi et al. 2010; Ghaziri et al. 2013). The results of our study could contribute to the improvement of treatment precision via tomographic neurofeedback (e.g., Congedo et al. 2004; Liechti et al. 2012) in order to specifically target certain EEG oscillations originating from the TPJ.
References
Aghababaei N, Mohammadtabar S, Saffarinia M (2014) Dirty Dozen vs. the H factor: comparison of the Dark triad and honesty-humility in prosociality, religiosity, and happiness. Personality Individ Differ 67:6–10. https://doi.org/10.1016/j.paid.2014.03.026
Andreoni J, Miller J (2002) Giving according to GARP: an experimental test of the consistency of preferences for altruism. Econometrica 70:737–753. https://doi.org/10.1111/1468-0262.00302
Ashton MC, Lee K (2009) The HEXACO-60: a short measure of the major dimensions of personality. J Pers Assess 91:340–345. https://doi.org/10.1080/00223890902935878
Baumgartner T, Gianotti LRR, Knoch D (2013) Who is honest and why: baseline activation in anterior insula predicts inter-individual differences in deceptive behavior. Biol Psychol 94:192–197. https://doi.org/10.1016/j.biopsycho.2013.05.018
Becker A, Deckers T, Dohmen T et al (2012) The relationship between economic preferences and psychological personality measures. Annu Rev Econom 4:453–478. https://doi.org/10.1146/annurev-economics-080511-110922
Cannon RL, Baldwin DR, Shaw TL et al (2012) Reliability of quantitative EEG (qEEG) measures and LORETA current source density at 30 days. Neurosci Lett 518:27–31. https://doi.org/10.1016/j.neulet.2012.04.035
Chapman LJ, Chapman JP (1987) The measurement of handedness. Brain Cogn 6:175–183. https://doi.org/10.1016/0278-2626(87)90118-7
Congedo M, Lubar J, Joffe D et al (2004) Low-resolution electromagnetic tomography neurofeedback. IEEE Trans Rehabil Eng Inst Electr Electron Eng 4:387–397. https://doi.org/10.1109/TNSRE.2004.840492
Decety J, Lamm C (2009) Empathy versus personal distress: recent evidence from social neuroscience. In: Decety J, Ickes W (eds) The social neuroscience of empathy. MIT Press, Cambridge, pp 199–214
Dünki RM, Schmid GB, Stassen HH (2000) Intraindividual specificity and stability of human EEG: comparing a linear vs a nonlinear approach. Methods Inf Med 39:78–82
Edwards A (1957) The social desirability variable in personality assessment and research. Dryden Press, New York
Epstein ZG, Peysakhovich A, Rand DG (2016) The good, the bad, and the unflinchingly selfish: cooperative decision-making can be predicted with high accuracy using only three behavioral types. SSRN Electron J. https://doi.org/10.2139/ssrn.2737983
Fermin ASR, Sakagami M, Kiyonari T et al (2016) Representation of economic preferences in the structure and function of the amygdala and prefrontal cortex. Sci Rep 6:20982. https://doi.org/10.1038/srep20982
Fischbacher U, Gächter S (2010) Social preferences, beliefs, and the dynamics of free riding in public goods experiments. Am Econ Rev 100:541–556
Ghaziri J, Tucholka A, Larue V et al (2013) Neurofeedback training induces changes in white and gray matter. Clin EEG Neurosci 44:265–272. https://doi.org/10.1177/1550059413476031
Gianotti LRR, Knoch D, Faber P et al (2009) Tonic activity level in the right prefrontal cortex predicts individuals’ risk taking. Psychol Sci 20:33–38. https://doi.org/10.1111/j.1467-9280.2008.02260.x
Gianotti LRR, Figner B, Ebstein RP, Knoch D (2012) Why some people discount more than others: baseline activation in the dorsal PFC mediates the link between COMT genotype and impatient choice. Front Neurosci 6:1–12. https://doi.org/10.3389/fnins.2012.00054
Hare TA, Camerer CF, Knoepfle DT et al (2010) Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J Neurosci 30:583–590. https://doi.org/10.1523/JNEUROSCI.4089-09.2010
Hilbig BE, Zettler I, Heydasch T (2012) Personality, punishment and public goods: strategic shifts towards cooperation as a matter of dispositional honesty-humility. Eur J Pers 26:245–254. https://doi.org/10.1002/per.830
Isaac RM, Walker JA (1988) Group size effects in public goods provision: the voluntary contributions. Q J Econ 103:179–199. https://doi.org/10.2307/1882648
Knoch D, Gianotti LRR, Baumgartner T, Fehr E (2010) A neural marker of costly punishment behavior. Psychol Sci 21:337–342. https://doi.org/10.1177/0956797609360750
Kubicki S, Hrrmann WM, Fichte K, Freund G (1979) Reflections on the topics: EEG frequency bands and regulation of vigilance. Pharmacopsychiatry 12:237–245. https://doi.org/10.1055/s-0028-1094615
Kurzban R, Houser D (2001) Individual differences in cooperation in a circular public goods game. Eur J Pers 15:37–52. https://doi.org/10.1002/per.420
Lamm C, Bukowski H, Silani G (2016) From shared to distinct self–other representations in empathy: evidence from neurotypical function and socio-cognitive disorders. Philos Trans R Soc B 371:20150083. https://doi.org/10.1098/rstb.2015.0083
Lancaster JL, Woldorff MG, Parsons LM, et al (2000) Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131. https://doi.org/10.1002/1097-0193(200007)10:3%3C120::AID-HBM30%3E3.0.CO;2-8
Lazar SW, Kerr CE, Wasserman RH et al (2005) Meditation experience is associated with increased cortical thickness. Neuroreport 16:1893–1897. https://doi.org/10.1097/01.wnr.0000186598.66243.19
Liechti MD, Maurizio S, Heinrich H et al (2012) First clinical trial of tomographic neurofeedback in attention-deficit/hyperactivity disorder: evaluation of voluntary cortical control. Clin Neurophysiol 123:1989–2005. https://doi.org/10.1016/j.clinph.2012.03.016
Lombardo MV, Chakrabarti B, Bullmore ET, Baron-Cohen S (2011) Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. Neuroimage 56:1832–1838. https://doi.org/10.1016/j.neuroimage.2011.02.067
Mazziotta J, Toga A, Evans A et al (2001) A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM). Philos Trans R Soc B 356:1293–1322. https://doi.org/10.1098/rstb.2001.0915
Mobascher A, Brinkmeyer J, Warbrick T et al (2009a) Fluctuations in electrodermal activity reveal variations in single trial brain responses to painful laser stimuli—a fMRI/EEG study. Neuroimage 44:1081–1092. https://doi.org/10.1016/j.neuroimage.2008.09.004
Mobascher A, Brinkmeyer J, Warbrick T et al (2009b) Laser-evoked potential P2 single-trial amplitudes covary with the fMRI BOLD response in the medial pain system and interconnected subcortical structures. Neuroimage 45:917–926. https://doi.org/10.1016/j.neuroimage.2008.12.051
Morishima Y, Schunk D, Bruhin A et al (2012) Linking brain structure and activation in temporoparietal junction to explain the neurobiology of human altruism. Neuron 75:73–79. https://doi.org/10.1016/j.neuron.2012.05.021
Näpflin M, Wildi M, Sarnthein J (2007) Test-retest reliability of resting EEG spectra validates a statistical signature of persons. Clin Neurophysiol 118:2519–2524. https://doi.org/10.1016/j.clinph.2007.07.022
Nash K, Gianotti LRR, Knoch D (2015) A neural trait approach to exploring individual differences in social preferences. Front Behav Neurosci 8:458. https://doi.org/10.3389/fnbeh.2014.00458
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25. https://doi.org/10.1002/hbm.1058
Nichols AL, Maner JK (2008) The good-subject effect: investigating participant demand characteristics. J Gen Psychol 135:151–166. https://doi.org/10.3200/GENP.135.2.151-166
Nuwer MR, Comi C, Emerson R et al (1998) IFCN standards for digitial recording of clinical EEG. Electroencephalogr Clin Neurophysiol 106:259–261. https://doi.org/10.1016/S0013-4694(97)00106-5
Pantelis PC, Byrge L, Tyszka JM et al (2015) A specific hypoactivation of right temporo-parietal junction/posterior superior temporal sulcus in response to socially awkward situations in autism. Soc Cogn Affect Neurosci 10:1348–1356. https://doi.org/10.1093/scan/nsv021
Park J, Kim H, Sohn J-W et al (2018) EEG beta oscillations in the temporoparietal area related to the accuracy in estimating others’ preference. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2018.00043
Pascual-Marqui RD (2002) Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol 24(Suppl D):5–12
Peysakhovich A, Nowak MA, Rand DG (2014) Humans display a ‘cooperative phenotype’ that is domain general and temporally stable. Nat Commun 5:4939. https://doi.org/10.1038/ncomms5939
Riecansky I, Paul N, Kölble S et al (2014) Beta EEG oscillations reveal ethnic ingroup bias in sensorimotor resonance to pain of others. Soc Cogn Affect Neurosci 54:1–9. https://doi.org/10.1111/j.1399-0012.2012.01641.x
Rullmann M, Anwander A, Dannhauer M et al (2009) EEG source analysis of epileptiform activity using a 1 mm anisotropic hexahedra finite element head model. Neuroimage 44:399–410. https://doi.org/10.1016/j.neuroimage.2008.09.009
Soutschek A, Ruff CC, Strombach T et al (2016) Brain stimulation reveals crucial role of overcoming self-centeredness in self-control. Sci Adv 2:e1600992–e1600992. https://doi.org/10.1126/sciadv.1600992
Steinbeis N (2016) The role of self-other distinction in understanding others’ mental and emotional states: neurocognitive mechanisms in children and adults. Philos Trans R Soc B 371:20150074. https://doi.org/10.1098/rstb.2015.0074
Strombach T, Weber B, Hangebrauk Z et al (2015) Social discounting involves modulation of neural value signals by temporoparietal junction. Proc Natl Acad Sci USA 112:1619–1624. https://doi.org/10.1073/pnas.1414715112
Studer B, Pedroni A, Rieskamp J (2013) Predicting risk-taking behavior from prefrontal resting-state activity and personality. PLoS ONE. https://doi.org/10.1371/journal.pone.0076861
Takeuchi H, Sekiguchi A, Taki Y et al (2010) Training of working memory impacts structural connectivity. J Neurosci 30:3297–3303. https://doi.org/10.1523/JNEUROSCI.4611-09.2010
Thielmann I, Hilbig BE (2014) Trust in me, trust in you: a social projection account of the link between personality, cooperativeness, and trustworthiness expectations. J Res Pers 50:61–65. https://doi.org/10.1016/j.jrp.2014.03.006
Watanabe T, Takezawa M, Nakawake Y et al (2014) Two distinct neural mechanisms underlying indirect reciprocity. Proc Natl Acad Sci 111:3990–3995. https://doi.org/10.1073/pnas.1318570111
Watson D, Clark LA, Tellegen A (1988) Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54:1063–1070. https://doi.org/10.1037/0022-3514.54.6.1063
Zhao K, Ferguson E, Smillie LD (2016) Prosocial personality traits differentially predict egalitarianism, generosity, and reciprocity in economic games. Front Psychol. https://doi.org/10.3389/fpsyg.2016.01137
Funding
This work was supported by grants from the Swiss National Science Foundation to D.K. and L.G. (100019_166006) and the Mens Sana Foundation to D.K.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all subjects included in the study.
Additional information
Handling Editor: Micah M. Murray.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gianotti, L.R.R., Dahinden, F.M., Baumgartner, T. et al. Understanding Individual Differences in Domain-General Prosociality: A Resting EEG Study. Brain Topogr 32, 118–126 (2019). https://doi.org/10.1007/s10548-018-0679-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-018-0679-y