Abstract
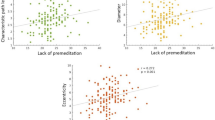
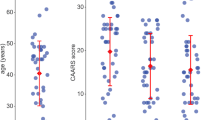
Impulsiveness is a central human personality trait and of high relevance for the development of several mental disorders. Impulsiveness is a multidimensional construct, yet little is known about dimension-specific neural correlates. Here, we address the question whether motor, attentional and non-planning components, as measured by the Barratt Impulsiveness Scale (BIS-11), are associated with distinct or overlapping neural network activity. In this study, we investigated brain activity at rest and its relationship to distinct dimensions of impulsiveness in 30 healthy young adults (m/f = 13/17; age mean/SD = 26.4/2.6 years) using resting-state functional magnetic resonance imaging at 3T. A spatial independent component analysis and a multivariate model selection strategy were used to identify systems loading on distinct impulsivity domains. We first identified eight networks for which we had a-priori hypotheses. These networks included basal ganglia, cortical motor, cingulate and lateral prefrontal systems. From the eight networks, three were associated with impulsiveness measures (p < 0.05, FDR corrected). There were significant relationships between right frontoparietal network function and all three BIS domains. Striatal and midcingulate network activity was associated with motor impulsiveness only. Within the networks regionally confined effects of age and gender were found. These data suggest distinct and overlapping patterns of neural activity underlying specific dimensions of impulsiveness. Motor impulsiveness appears to be specifically related to striatal and midcingulate network activity, in contrast to a domain-unspecific right frontoparietal system. Effects of age and gender have to be considered in young healthy samples.


Similar content being viewed by others
References
Achterberg M et al (2016) Control your anger! The neural basis of aggression regulation in response to negative social feedback. Soc Cogn Affect Neurosci 11:712–720
Adinoff B et al (2006) Sex differences in medial and lateral orbitofrontal cortex hypoperfusion in cocaine-dependent men and women. Gend Med 3:206–222
Aichert DS et al (2012) Associations between trait impulsivity and prepotent response inhibition. J Clin Exp Neuropsychol 34:1016–1032
Allen EA et al (2011) A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci 5:2
Aron AR (2011) From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry 69:e55–e68
Aron AR, Poldrack RA (2005) The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1285–1292
Aron AR, Poldrack RA (2006) Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci 26:2424–2433
Aron AR et al (2003) Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 6:115–116
Aron AR, Robbins TW, Poldrack RA (2004) Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8:170–177
Asahi S et al (2004) Negative correlation between right prefrontal activity during response inhibition and impulsiveness: a fMRI study. Eur Arch Psychiatry Clin Neurosci 254:245–251
Bari A, Robbins TW (2013) Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108:44–79
Bernstein GA et al (2016) Abnormal striatal resting-state functional connectivity in adolescents with obsessive-compulsive disorder. Psychiatry Res 247:49–56
Biswal BB et al (2010) Toward discovery science of human brain function. Proc Natl Acad Sci USA 107:4734–4739
Blakemore SJ, Robbins TW (2012) Decision-making in the adolescent brain. Nat Neurosci 15:1184–1191
Boehler CN et al (2012) Motivating inhibition—reward prospect speeds up response cancellation. Cognition 125:498–503
Brown MR et al (2015a) fMRI investigation of response inhibition, emotion, impulsivity, and clinical high-risk behavior in adolescents. Front Syst Neurosci 9:124
Brown MR et al (2015b) Neural correlates of high-risk behavior tendencies and impulsivity in an emotional Go/NoGo fMRI task. Front Syst Neurosci 9:24
Chamberlain SR et al (2007) Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry 164:335–338
Chambers RA, Potenza MN (2003) Neurodevelopment, impulsivity, and adolescent gambling. J Gambl Stud 19:53–84
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215
Correa N et al (2005) Comparison of blind source separation algorithms for FMRI using a new Matlab toolbox: GIFT. Proc IEEE Int Conf Acoust Speech Signal Process 5:401–404
Cuthbert BN, Insel TR (2013) Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 11:126
Cyders MA, Coskunpinar A (2011) Measurement of constructs using self-report and behavioral lab tasks: is there overlap in nomothetic span and construct representation for impulsivity? Clin Psychol Rev 31:965–982
Davis FC et al (2013) Impulsivity and the modular organization of resting-state neural networks. Cereb Cortex 23:1444–1452
de Wit SJ et al (2012) Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry 169:1100–1108
Delgado MR, Gillis MM, Phelps EA (2008) Regulating the expectation of reward via cognitive strategies. Nat Neurosci 11:880–881
Eysenck SB, Eysenck HJ (1978) Impulsiveness and venturesomeness: their position in a dimensional system of personality description. Psychol Rep 43:1247–1255
Farr OM et al (2012) Decreased saliency processing as a neural measure of Barratt impulsivity in healthy adults. Neuroimage 63:1070–1077
Fassbender C et al (2004) A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Res Cogn Brain Res 20:132–143
Fineberg NA et al (2014) New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr 19:69–89
Fuster J (2008) The prefrontal cortex. Academic Press, London
Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669
Hariri AR et al (2006) Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci 26:13213–13217
Hester R, Fassbender C, Garavan H (2004) Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex 14:986–994
Himberg J, Hyvarinen A, Esposito F (2004) Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 22:1214–1222
Hirjak D et al (2016) Cortical folding patterns are associated with impulsivity in healthy young adults. Brain Imaging Behav. https://doi.org/10.1007/s11682-016-9618-2
Horn NR et al (2003) Response inhibition and impulsivity: an fMRI study. Neuropsychologia 41:1959–1966
Kim S, Lee D (2011) Prefrontal cortex and impulsive decision making. Biol Psychiatry 69:1140–1146
Kirby KN (2009) One-year temporal stability of delay-discount rates. Psychon Bull Rev 16:457–462
Knutson B et al (2001) Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12:3683–3687
Konishi S et al (1999) Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain 122(Pt 5):981–991
Laird AR et al (2011) Behavioral interpretations of intrinsic connectivity networks. J Cognitive Neurosci 23(12):4022–4037
Lei D et al (2015) Functional MRI reveals different response inhibition between adults and children with ADHD. Neuropsychology 29:874–881
Levy R, Goldman-Rakic PS (2000) Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res 133:23–32
Li YO, Adali T, Calhoun VD (2007) Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp 28:1251–1266
Li N et al (2013) Resting-state functional connectivity predicts impulsivity in economic decision-making. J Neurosci 33:4886–4895
Lijffijt M et al (2004) Differences between low and high trait impulsivity are not associated with differences in inhibitory motor control. J Atten Disord 8:25–32
Liu J, Zubieta JK, Heitzeg M (2012) Sex differences in anterior cingulate cortex activation during impulse inhibition and behavioral correlates. Psychiatry Res 201:54–62
Logan TF et al (1997) Biologic response modulation by tumor necrosis factor alpha (TNF alpha) in a phase Ib trial in cancer patients. J Immunother 20:387–398
Matsuo K et al (2009) A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp 30:1188–1195
McClure SM et al (2004) Separate neural systems value immediate and delayed monetary rewards. Science 306:503–507
Meyer-Lindenberg A et al (2006) Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA 103:6269–6274
Milad MR, Rauch SL (2012) Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci 16:43–51
Milad MR et al (2013) Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry 70:608–618 (quiz 554)
Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202
Moeller FG et al (2001) Psychiatric aspects of impulsivity. Am J Psychiatry 158:1783–1793
Nambu A, Tokuno H, Takada M (2002) Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res 43:111–117
Oldehinkel M et al (2016) Attention-deficit/hyperactivity disorder symptoms coincide with altered striatal connectivity. Biol Psychiatry 1:353–363
Paus T et al (1993) Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol 70:453–469
Peters J, Buchel C (2011) The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn Sci 15:227–239
Pietrini P et al (2000) Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. Am J Psychiatry 157:1772–1781
Plichta MM, Scheres A (2014) Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev 38:125–134
Posner J, Park C, Wang Z (2014) Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol Rev 24:3–15
Power JD et al (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154
Rubia K et al (2001) Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage 13:250–261
Schilling C et al (2013) Cortical thickness of superior frontal cortex predicts impulsiveness and perceptual reasoning in adolescence. Mol Psychiatry 18:624–630
Schmaal L et al (2012) The association between cingulate cortex glutamate concentration and delay discounting is mediated by resting state functional connectivity. Brain Behav 2:553–562
Smith SM et al (2009) Correspondence of the brain’s functional architecture during activation and rest. P Natl Acad Sci 106(31):13040–13045
Stanford MS et al (2009) Fifty years of the Barratt Impulsiveness Scale: an update and review. Personality Individ Differ 47:385–395
Stein DJ, Hollander E (1995) Obsessive-compulsive spectrum disorders. J Clin Psychiatry 56:265–266
Stoltenberg SF (2008) Does gender moderate associations among impulsivity and health-risk behaviors? Addict Behav 33(2):252–265
Tschernegg M et al (2015) Impulsivity relates to striatal gray matter volumes in humans: evidence from a delay discounting paradigm. Front Hum Neurosci 9:384
Tzourio-Mazoyer N et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Urcelay GP, Dalley JW (2012) Linking ADHD, impulsivity, and drug abuse: a neuropsychological perspective. Curr Top Behav Neurosci 9:173–197
Whelan R et al (2012) Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci 15:920–925
Wilbertz T et al (2014) Response inhibition and its relation to multidimensional impulsivity. Neuroimage 103:241–248
Yip SW, Potenza MN (2016) Application of research domain criteria to childhood and adolescent impulsive and addictive disorders: implications for treatment. Clin Psychol Rev. https://doi.org/10.1016/j.cpr.2016.11.003
Acknowledgements
We are grateful to all the participants and their families for their time and interest in this study.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Ethical Approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10548_2017_604_MOESM2_ESM.tif
Supplementary Figure 1. Spatiotemporal patterns of eight resting-state networks chosen for further multivariate analyses to investigate neural systems loadings on distinct domains of impulsiveness, as provided by the BIS-11 scale. The figures displays independent components (ICs) and their corresponding time courses, as identified by the group ICA. The color bars indicate Z-values, IC’s are thresholded above Z = 3.5. (TIF 13463 KB)
10548_2017_604_MOESM3_ESM.tif
Supplementary Figure 2. Univariate tests showing regional effects of age and gender over all resting-state networks, displayed as −sign(t)log10(p). Stereotaxic coordinates and Z-scores are provided in Table 1, supplementary data. Effects were considered significant at p < 0.01, uncorrected. The panels show bar plots of average beta-values for age and gender terms, respectively. Beta-values were averaged over significant clusters showing the same directionality. The color of the bar is proportional to the fraction of voxels within components that contribute to each of the effects. (TIF 102 KB)
Rights and permissions
About this article
Cite this article
Kubera, K.M., Hirjak, D., Wolf, N.D. et al. Intrinsic Network Connectivity Patterns Underlying Specific Dimensions of Impulsiveness in Healthy Young Adults. Brain Topogr 31, 477–487 (2018). https://doi.org/10.1007/s10548-017-0604-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-017-0604-9