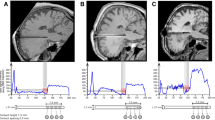
Abstract
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is nowadays an evidence-based state of the art therapy option for motor and non-motor symptoms in patients with Parkinson’s disease (PD). However, the exact anatomical regions of the cerebral network that are targeted by STN–DBS have not been precisely described and no definitive pre-intervention predictors of the clinical response exist. In this study, we test the hypothesis that the clinical effectiveness of STN–DBS depends on the connectivity profile of the targeted brain networks. Therefore, we used diffusion-weighted imaging (DWI) and probabilistic tractography to reconstruct the anatomical networks and the graph theoretical framework to quantify the connectivity profile. DWI was obtained pre-operatively from 15 PD patients who underwent DBS (mean age = 67.87 ± 7.88, 11 males, H&Y score = 3.5 ± 0.8) using a 3T MRI scanner (Philips Achieva). The pre-operative connectivity properties of a network encompassing frontal, prefrontal cortex and cingulate gyrus were directly linked to the postoperative clinical outcome. Eccentricity as a topological-characteristic of the network defining how cerebral regions are embedded in relation to distant sites correlated inversely with the applied voltage at the active electrode for optimal clinical response. We found that network topology and pre-operative connectivity patterns have direct influence on the clinical response to DBS and may serve as important and independent predictors of the postoperative clinical outcome.





Similar content being viewed by others
Abbreviations
- AAL:
-
Automated anatomical labeling
- AUC:
-
Area under the curve
- BCT:
-
Brain connectivity toolbox
- COG:
-
Center of gravity
- DBS:
-
Deep brain stimulation
- DWI:
-
Diffusion-weighted imaging
- FWHM:
-
Full width at half maximum
- H & Y:
-
Hoehn and Yahr
- MED OFF/ON:
-
Medication off/on
- MPRAGE:
-
Magnetization-prepared rapid gradient-echo
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- SMA:
-
Supplementary motor area
- STN:
-
Subthalamic nucleus
- UPDRS:
-
Unified Parkinson’s disease rating scale
- VTA:
-
Volume of tissue activation
References
Achard S, Bullmore E (2007) Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3(2):e17
Amboni M, Cozzolino A, Longo K, Picillo M, Barone P (2008) Freezing of gait and executive functions in patients with Parkinson’s disease. Mov Disord 23(3):395–400
Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34(1):144–155
Blondel VD, Guillaume JL, Lambiotte R, Lefebvre E (2008) Fast unfolding of communities in large networks. J Stat Mech 2008(10):10008
Brittain JS, Brown P (2014) Oscillations and the basal ganglia: motor control and beyond. Neuroimage 85:637–647
Brunenberg EJ, Moeskops P, Backes WH, Pollo C, Cammoun L, Vilanova A et al (2012a) Structural and resting state functional connectivity of the subthalamic nucleus: identification of motor STN parts and the hyperdirect pathway. PloS ONE 7(6):e39061
Brunenberg EJL, Moeskops P, Backes WH, Pollo C, Cammoun L, Vilanova A et al (2012b) Structural and resting state functional connectivity of the subthalamic nucleus: identification of motor STN parts and the hyperdirect pathway. PloS ONE 7(6):e39061
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10(3):186–198
Butson CR, Cooper SE, Henderson JM, McIntyre CC (2007) Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage 34(17113789):661–670
Canu E, Agosta F, Sarasso E, Volonte MA, Basaia S, Stojkovic T et al (2015) Brain structural and functional connectivity in Parkinson’s disease with freezing of gait. Hum Brain Mapp 36(12):5064–5078
Fukaya C, Yamamoto T (2015) Deep brain stimulation for parkinson’s disease: recent trends and future direction. Neurologia Medico-Chirurgica 55(5):422–431
Girvan M, Newman MEJ (2002) Community structure in social and biological networks. Proc Natl Acad Sci USA 99(12):7821–7826
Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K (2009) Optical deconstruction of parkinsonian neural circuitry. Science 324(5925):354–359
Groppa S, Herzog J, Falk D, Riedel C, Deuschl G, Volkmann J (2014) Physiological and anatomical decomposition of subthalamic neurostimulation effects in essential tremor. Brain 137(Pt 1):109–121
Haynes WI, Haber SN (2013) The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J Neurosci 33(11):4804–4814
Herzog J, Fietzek U, Hamel W, Morsnowski A, Steigerwald F, Schrader B et al (2004) Most effective stimulation site in subthalamic deep brain stimulation for Parkinson’s disease. Mov Disord 19(9):1050–1054
Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5(2):143–156
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17(2):825–841
Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM et al (2004) Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA 101(36):13335–13340
Klingelhoefer L, Samuel M, Chaudhuri KR, Ashkan K (2014) An update of the impact of deep brain stimulation on non motor symptoms in Parkinson’s disease. J Parkinson’s Dis 4(2):289–300
Koshimori Y, Segura B, Christopher L, Lobaugh N, Duff-Canning S, Mizrahi R et al (2015) Imaging changes associated with cognitive abnormalities in Parkinson’s disease. Brain Struct Funct 220(4):2249–2261
Latora V, Marchiori M (2001) Efficient behavior of small-world networks. Phys Rev Lett 87(19):198701
Li Q, Ke Y, Chan DCW, Qian ZM, Yung KKL, Ko H et al (2012) Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron 76(5):1030–1041
Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M (2008) et al. Disrupted small-world networks in schizophrenia. Brain 131(Pt 4):945–961
McIntyre CC, Hahn PJ (2010) Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis 38(3):329–337
McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL (2004) Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clinical Neurophysiol 115(3):589–595
Meunier D, Achard S, Morcom A, Bullmore E (2009) Age-related changes in modular organization of human brain functional networks. Neuroimage 44(3):715–723
Micheloyannis S, Pachou E, Stam CJ, Vourkas M, Erimaki S, Tsirka V (2006) Using graph theoretical analysis of multi channel EEG to evaluate the neural efficiency hypothesis. Neurosci Lett 402(3):273–277
Muthuraman M, Deuschl G, Koirala N, Riedel C, Volkmann J, Groppa S (2017) Effects of DBS in parkinsonian patients depend on the structural integrity of frontal cortex. Scientific Rep 7:43571
Nagano-Saito A, Washimi Y, Arahata Y, Kachi T, Lerch J, Evans A et al (2005) Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 64(2):224–229
Nambu A, Chiken S (2015) Mechanism of DBS: inhibition, Excitation, or Disruption? In: Itakura T (ed) Deep brain stimulation for neurological disorders: theoretical background and clinical application. Springer International Publishing, Cham, pp 13–20
Newman MEJ (2006) Modularity and community structure in networks. Proc Natl Acad Sci USA 103(23):8577–8582
Odekerken VJJ, van Laar T, Staal MJ, Mosch A, Hoffmann CFE, Nijssen PCG et al (2013) Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 12(1):37–44
Olde Dubbelink KT, Hillebrand A, Stoffers D, Deijen JB, Twisk JW, Stam CJ et al (2014) Disrupted brain network topology in Parkinson’s disease: a longitudinal magnetoencephalography study. Brain 137(Pt 1):197–207
Opsahl T, Colizza V, Panzarasa P, Ramasco JJ (2008) Prominence and control: the weighted rich-club effect. Phys Rev Lett 101:168702
Park H-J, Friston K (2013) Structural and functional brain networks: from connections to cognition. Science 342(6158):1238411
Pavlopoulos GA, Secrier M, Moschopoulos CN, Soldatos TG, Kossida S, Aerts J et al (2011) Using graph theory to analyze biological networks. BioData Mining 4:10
Pereira JB, Ibarretxe-Bilbao N, Marti M-J, Compta Y, Junqué C, Bargallo N et al (2012) Assessment of cortical degeneration in patients with Parkinson’s disease by voxel-based morphometry, cortical folding, and cortical thickness. Hum Brain Mapp 33(11):2521–2534
Pereira JB, Aarsland D, Ginestet CE, Lebedev AV, Wahlund LO, Simmons A et al. (2015) Aberrant cerebral network topology and mild cognitive impairment in early Parkinson’s disease. Hum Brain Mapp 36(8):2980–2995
Ranck JB (1975) Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98(3):417–440
Ritchey M, Yonelinas AP, Ranganath C (2014) Functional connectivity relationships predict similarities in task activation and pattern information during associative memory encoding. J Cogn Neurosci 26(5):1085–1099
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52(3):1059–1069
Skidmore F, Korenkevych D, Liu Y, He G, Bullmore E, Pardalos PM (2011) Connectivity brain networks based on wavelet correlation analysis in Parkinson fMRI data. Neurosci Lett 499(1):47–51
Sporns O (2003) Graph theory methods for the analysis of neural connectivity patterns. In neuroscience databases. Springer, New York, pp. 171–185
Sporns O, Zwi JD (2004) The small world of the cerebral cortex. Neuroinformatics 2(2):145–162
Stam CJ (2004) Functional connectivity patterns of human magnetoencephalographic recordings: a ‘small-world’ network? Neurosci Lett 355(1–2):25–28
Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P (2007) Small-world networks and functional connectivity in Alzheimer’s disease. Cereb Cortex 17(1):92–99
Stam CJ, de Haan W, Daffertshofer A, Jones BF, Manshanden I, van Cappellen van Walsum AM et al (2009) Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer’s disease. Brain 132(1):213–224
Stein E, Bar-Gad I (2013) Beta oscillations in the cortico-basal ganglia loop during parkinsonism. Exp Neurol 245:52–59
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1):273–289
Udupa K, Chen R (2015) The mechanisms of action of deep brain stimulation and ideas for the future development. Prog Neurobiol 133:27–49
van Straaten ECW, Stam CJ (2013) Structure out of chaos: functional brain network analysis with EEG, MEG, and functional MRI. Eur Neuropsychopharmacol 23(1):7–18
van den Heuvel MP, Sporns O (2011) Rich-Club organization of the human connectome. J Neurosci 31(44):15775–15786
van den Heuvel MP, Stam CJ, Kahn RS, Pol HE (2009) Efficiency of functional brain networks and intellectual performance. J Neurosci 29(23):7619–7624
Vanegas-Arroyave N, Lauro PM, Huang L, Hallett M, Horovitz SG, Zaghloul KA et al. (2016) Tractography patterns of subthalamic nucleus deep brain stimulation. Brain 139(4), 1200–1210.
Voges J, Volkmann J, Allert N, Lehrke R, Koulousakis A, Freund HJ et al. (2002) Bilateral high-frequency stimulation in the subthalamic nucleus for the treatment of Parkinson disease: correlation of therapeutic effect with anatomical electrode position. J Neurosurg 96(2):269–279
Weaver FM, Follett KA, Stern M, Luo P, Harris CL, Hur K et al (2012) Randomized trial of deep brain stimulation for Parkinson disease Thirty-six-month outcomes. Neurology 79(1):55–65
Witt K, Granert O, Daniels C, Volkmann J, Falk D, van Eimeren T et al (2013) Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson’s disease: results from a randomized trial. Brain 136(7):2109–2119
Wodarg F, Herzog J, Reese R, Falk D, Pinsker MO, Steigerwald F et al (2012) Stimulation site within the MRI-defined STN predicts postoperative motor outcome. Mov Disord 27(7):874–879
Yu Q, Sui J, Rachakonda S, He H, Gruner W, Pearlson G et al (2011) Altered topological properties of functional network connectivity in schizophrenia during resting state: a small-world brain network study. PloS ONE 6(9):e25423
Acknowledgements
This work was supported by the German Research Foundation (DFG; CRC-TR-128).
Author information
Authors and Affiliations
Corresponding author
Additional information
Muthuraman Muthuraman and Sergiu Groppa have contributed equally.
Rights and permissions
About this article
Cite this article
Koirala, N., Fleischer, V., Glaser, M. et al. Frontal Lobe Connectivity and Network Community Characteristics are Associated with the Outcome of Subthalamic Nucleus Deep Brain Stimulation in Patients with Parkinson’s Disease. Brain Topogr 31, 311–321 (2018). https://doi.org/10.1007/s10548-017-0597-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-017-0597-4