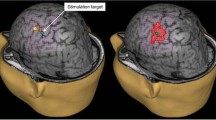
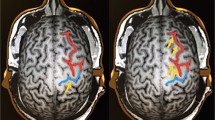
Abstract
This is an explorative study applying presurgical navigated transcranial magnetic stimulation (nTMS) to investigate the spatial distributions of motor sites to reveal tumor-induced brain plasticity in patients with brain tumors. We analyzed nTMS-based motor maps derived from presurgical mapping of 100 patients with motor eloquently located brain tumors (tumors in the frontal lobe, the precentral gyrus [PrG], the postcentral gyrus [PoG], the remaining parietal lobe, or the temporal lobe). Based on these motor maps, we systematically investigated changes in motor evoked potential (MEP) counts among 4 gyri (PrG, PoG, medial frontal gyrus, and superior frontal gyrus) between subgroups of patients according to the tumor location in order to depict the tumor’s influence on reorganization. When comparing patients with different tumor locations, high MEP counts were elicited less frequently by stimulating the PrG in patients with tumors directly affecting the PrG (p < 0.05). Still, in more than 50% of these patients, the MEP counts elicited by stimulating the PrG were higher than average, indicating robust motor representations within the primary motor cortex. In contrast, patients with PoG and parietal tumors primarily showed high MEP counts when stimulating the PoG (p < 0.10). The functional reorganization is not likely to induce a shift of motor function from the PrG to adjacent regions but rather leads to a reorganization within anatomical constraints, such as of the PoG. Thus, presurgical nTMS-based motor mapping sensitively depicted the tumor-induced plasticity of the motor cortex.




Similar content being viewed by others
Abbreviations
- 3D:
-
Three-dimensional
- ADM:
-
Abductor digiti minimi muscle
- ANOVA:
-
Analysis of variance
- APB:
-
Abductor pollicis brevis muscle
- BMRC:
-
British Medical Research Council
- DCS:
-
Direct cortical stimulation
- EMG:
-
Electromyography
- FCR:
-
Flexor carpi radialis muscle
- HGG:
-
High-grade glioma
- LGG:
-
Low-grade glioma
- MEP:
-
Motor evoked potential
- MFG:
-
Middle frontal gyrus
- MRI:
-
Magnetic resonance imaging
- nTMS:
-
Navigated transcranial magnetic stimulation
- PMC:
-
Premotor cortex
- PoG:
-
Postcentral gyrus
- PrG:
-
Precentral gyrus
- rMT:
-
Resting motor threshold
- SFG:
-
Superior frontal gyrus
- UE:
-
Upper extremity
References
Almairac F, Herbet G, Moritz-Gasser S, Duffau H (2014) Parietal network underlying movement control: disturbances during subcortical electrostimulation. Neurosurg Rev 37:513–516. doi:10.1007/s10143-014-0530-1 (discussion 516-517)
Bulubas L et al (2016) Motor areas of the frontal cortex in patients with motor eloquent brain lesions. J Neurosurg 125:1431–1442. doi:10.3171/2015.11.JNS152103
Capelle L et al (2013) Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J Neurosurg 118:1157–1168. doi:10.3171/2013.1.JNS121
Cicinelli P, Traversa R, Rossini PM (1997) Post-stroke reorganization of brain motor output to the hand: a 2–4 month follow-up with focal magnetic transcranial stimulation. Electroencephalogr Clin Neurophysiol 105:438–450
Conway N et al (2017) Cortical plasticity of motor-eloquent areas measured by navigated transcranial magnetic stimulation in patients with glioma. J Neurosurg:1–11 doi:10.3171/2016.9.JNS161595
Desmurget M, Bonnetblanc F, Duffau H (2007) Contrasting acute and slow-growing lesions: a new door to. Brain Plast Brain 130:898–914. doi:10.1093/brain/awl300
Duffau H (2005) Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol 4:476–486. doi:10.1016/S1474-4422(05)70140-X
Duffau H (2006) Brain plasticity: from pathophysiological mechanisms to therapeutic applications. J Clin Neurosci 13:885–897. doi:10.1016/j.jocn.2005.11.045
Duffau H (2014) The huge plastic potential of adult brain and the role of connectomics: new insights provided by serial mappings in glioma surgery. Cortex 58:325–337. doi:10.1016/j.cortex.2013.08.005
Duffau H, Denvil D, Capelle L (2002) Long term reshaping of language, sensory, and motor maps after glioma resection: a new parameter to integrate in the surgical strategy. J Neurol Neurosurg Psychiatr 72:511–516
Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG (2004) Reorganization of the human ipsilesional premotor cortex after stroke. Brain 127:747–758. doi:10.1093/brain/awh082
Hayashi Y, Nakada M, Kinoshita M, Hamada J (2014) Functional reorganization in the patient with progressing glioma of the pure primary motor cortex: a case report with special reference to the topographic central sulcus defined by somatosensory-evoked potential. World Neurosurg 82:536 e531–534 doi:10.1016/j.wneu.2013.01.084
Herbet G, Maheu M, Costi E, Lafargue G, Duffau H (2016) Mapping neuroplastic potential in brain-damaged patients. Brain 139:829–844. doi:10.1093/brain/awv394
Ius T, Angelini E, Thiebaut de Schotten M, Mandonnet E, Duffau H (2011) Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a “minimal common brain”. Neuroimage 56:992–1000. doi:10.1016/j.neuroimage.2011.03.022
Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA (2003) The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis 12:119–126. doi:10.1016/s1052-3057(03)00042-9
Kombos T, Suess O, Kern BC, Funk T, Hoell T, Kopetsch O, Brock M (1999) Comparison between monopolar and bipolar electrical stimulation of the motor cortex. Acta Neurochir 141:1295–1301. doi:10.1007/s007010050433
Krieg SM, Shiban E, Buchmann N, Gempt J, Foerschler A, Meyer B, Ringel F (2012) Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. J Neurosurg 116:994–1001. doi:10.3171/2011.12.JNS111524
Krieg SM et al (2015) Changing the clinical course of glioma patients by preoperative motor mapping with navigated transcranial magnetic brain stimulation. BMC Cancer 15:231. doi:10.1186/s12885-015-1258-1
Krieg SM et al (2017) Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir. doi:10.1007/s00701-017-3187-z
Liu Y, Rouiller EM (1999) Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys. Exp Brain Res 128:149–159
Mesulam MM (1998) From sensation to cognition. Brain 121(6):1013–1052
Moser T et al (2016) Resection of nTMS-positive prerolandic motor areas causes permanent impairment of motor function. Neurosurgery. doi:10.1093/neuros/nyw169
Pascual-Leone A et al (2011) Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr 24:302–315. doi:10.1007/s10548-011-0196-8
Picht T et al (2011) Preoperative functional mapping for rolandic brain tumor surgery: comparison of navigated transcranial magnetic stimulation to direct. Cortic Stimul Neurosurg 69:581–588. doi:10.1227/NEU.0b013e3182181b89 (discussion 588)
Picht T, Strack V, Schulz J, Zdunczyk A, Frey D, Schmidt S, Vajkoczy P (2012) Assessing the functional status of the motor system in brain tumor patients using transcranial magnetic stimulation. Acta Neurochir 154:2075–2081. doi:10.1007/s00701-012-1494-y
Robles SG, Gatignol P, Lehericy S, Duffau H (2008) Long-term brain plasticity allowing a multistage surgical approach to World Health Organization Grade II gliomas in eloquent areas. J Neurosurg 109:615–624. doi:10.3171/JNS/2008/109/10/0615
Rosenstock T, Grittner U, Acker G, Schwarzer V, Kulchytska N, Vajkoczy P, Picht T (2016) Risk stratification in motor area-related glioma surgery based on navigated transcranial magnetic stimulation data. J Neurosurg. doi:10.3171/2016.4.JNS152896
Rossini PM et al (1994) Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91:79–92
Rossini PM et al (2015) Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126:1071–1107. doi:10.1016/j.clinph.2015.02.001
Saisanen L et al (2008) Motor potentials evoked by navigated transcranial magnetic stimulation in healthy subjects. J Clin Neurophysiol 25:367–372. doi:10.1097/WNP.0b013e31818e7944
Seitz RJ, Huang Y, Knorr U, Tellmann L, Herzog H, Freund HJ (1995) Large-scale plasticity of the human motor cortex. Neuroreport 6:742–744
Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ (1998) Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol 55:1081–1088
Sollmann N et al (2017a) Clinical factors underlying the inter-individual variability of the resting motor threshold in navigated transcranial magnetic stimulation motor mapping. Brain Topogr 30:98–121. doi:10.1007/s10548-016-0536-9)
Sollmann N, Bulubas L, Tanigawa N, Zimmer C, Meyer B, Krieg SM (2017b) The variability of motor evoked potential latencies in neurosurgical motor mapping by preoperative navigated transcranial magnetic stimulation. BMC Neurosci 18:5. doi:10.1186/s12868-016-0321-4)
Southwell DG, Hervey-Jumper SL, Perry DW, Berger MS (2016) Intraoperative mapping during repeat awake craniotomy reveals the functional plasticity of adult cortex. J Neurosurg 124:1460–1469. doi:10.3171/2015.5.JNS142833
Tarapore PE, Tate MC, Findlay AM, Honma SM, Mizuiri D, Berger MS, Nagarajan SS (2012) Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J Neurosurg 117:354–362. doi:10.3171/2012.5.JNS112124
Tarapore PE et al (2016a) Safety and tolerability of navigated TMS for preoperative mapping in neurosurgical patients. Clin Neurophysiol 127:1895–1900. doi:10.1016/j.clinph.2015.11.042 a)
Tarapore PE et al (2016b) Safety and tolerability of navigated TMS in healthy volunteers. Clin. neurophysiol. 127:1916–1918. doi:10.1016/j.clinph.2015.11.043 b)
Teitti S et al (2008) Non-primary motor areas in the human frontal lobe are connected directly to hand muscles. Neuroimage 40:1243–1250 doi:10.1016/j.neuroimage.2007.12.065
Uematsu S et al (1992) Motor and sensory cortex in humans: topography studied with chronic subdural stimulation. Neurosurgery 31:59–71 (discussion 71-52)
van Geemen K, Herbet G, Moritz-Gasser S, Duffau H (2014) Limited plastic potential of the left ventral premotor cortex in speech articulation: evidence from intraoperative awake mapping in glioma patients. Hum Brain Mapp 35:1587–1596. doi:10.1002/hbm.22275
Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS (1993) Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol 33:181–189. doi:10.1002/ana.410330208
Wunderlich G, Knorr U, Herzog H, Kiwit JC, Freund HJ, Seitz RJ (1998) Precentral glioma location determines the displacement of cortical hand representation. Neurosurgery 42:18–26 (discussion 26-17)
Acknowledgements
LB and NS gratefully acknowledge the support of the Graduate School’s Faculty Graduate Center of Medicine at our university. Moreover, the study was supported by grants of the Wilhelm Sander foundation.
Funding
The study was financed by institutional grants from the Department of Neurosurgery, the Section of Neuroradiology, and the Wilhelm Sander foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SK and BM are consultants for Brainlab AG (Munich, Germany). SK is a consultant for Nexstim Plc. (Helsinki, Finland). The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Bulubas, L., Sollmann, N., Tanigawa, N. et al. Reorganization of Motor Representations in Patients with Brain Lesions: A Navigated Transcranial Magnetic Stimulation Study. Brain Topogr 31, 288–299 (2018). https://doi.org/10.1007/s10548-017-0589-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-017-0589-4