Abstract
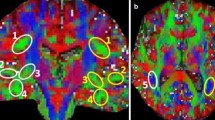
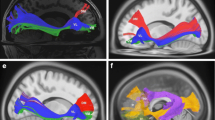
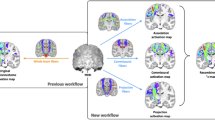
Diffusion tensor imaging (DTI) tractography and functional magnetic resonance imaging (fMRI) are powerful techniques to elucidate the anatomical and functional aspects of brain connectivity. However, integrating these approaches to describe the precise link between structure and function within specific brain circuits remains challenging. In this study, a novel DTI–fMRI integration method is proposed, to provide the topographical characterization and the volumetric assessment of the functional and anatomical connections within the language circuit. In a group of 21 healthy elderly subjects (mean age 68.5 ± 5.8 years), the volume of connection between the cortical activity elicited by a verbal fluency task and the cortico-cortical fiber tracts associated with this function are mapped and quantified. An application of the method to a case study in neuro-rehabilitation context is also presented. Integrating structural and functional data within the same framework, this approach provides an overall view of white and gray matter when studying specific brain circuits.




Similar content being viewed by others
References
Aslan S, Huang H, Uh J, Mishra V, Xiao G, van Osch MJ, Lu H (2011) White matter cerebral blood flow is inversely correlated with structural and functional connectivity in the human brain. Neuroimage 56:1145–1153
Baglio F, Griffanti L, Saibene FL et al (2014) Multistimulation group therapy in Alzheimer’s disease promotes changes in brain functioning. Neurorehabil Neural Repair. doi:10.1177/1545968314532833
Basho S, Palmer ED, Rubio MA, Wulfeck B, Müller R (2007) Effects of generation mode in fMRI adaptations of semantic fluency: paced production and overt speech. Neuropsychologia 45:1697–1706
Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A (2000) In vivo fiber tractography using DT-MRI data. Magn Reson Med 44:625–632
Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T (1997) Human brain language areas identified by functional magnetic resonance imaging. J Neurosci 17:353–362
Bonzano L, Pardini M, Mancardi GL, Pizzorno M, Roccatagliata L (2009) Structural connectivity influences brain activation during PVSAT in multiple sclerosis. Neuroimage 44:9–15
Catani M, de Schotten MT (2008) A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132
Catani M, Howard RJ, Pajevic S, Jones DK (2002) Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17:77–94
Catani M, Dell’Acqua F, Vergani F, Malik F, Hodge H, Roy P, Valabregue R, de Schotten MT (2012) Short frontal lobe connections of the human brain. Cortex 48:273–291
Ciccarelli O, Catani M, Johansen-Berg H, Clark C, Thompson A (2008) Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol 7:715–727
Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME (1999) Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci USA 96:10422–10427
Drobyshevsky A, Baumann SB, Schneider W (2006) A rapid fMRI task battery for mapping of visual, motor, cognitive, and emotional function. Neuroimage 31:732–744
Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WP, Ridderinkhof KR (2008) Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J Neurosci 28:9790–9796. doi:10.1523/JNEUROSCI.1465-08.2008
Friston KJ, Holmes AP, Worsley KJ, Poline J, Frith CD, Frackowiak RS (1994) Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2:189–210
Gimenez M, Junque C, Narberhaus A, Botet F, Bargallo N, Mercader JM (2006) Correlations of thalamic reductions with verbal fluency impairment in those born prematurely. NeuroReport 17:463–466. doi:10.1097/01.wnr.0000209008.93846.24
Glenn OA, Ludeman NA, Berman JI et al (2007) Diffusion tensor MR imaging tractography of the pyramidal tracts correlates with clinical motor function in children with congenital hemiparesis. AJNR Am J Neuroradiol 28:1796–1802. doi:10.3174/ajnr.A0676
Grefkes C, Fink GR (2011) Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain 134(Pt 5):1264–1276. doi:10.1093/brain/awr033
Greicius MD, Supekar K, Menon V, Dougherty RF (2009) Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19:72–78. doi:10.1093/cercor/bhn059
Greve DN, Fischl B (2009) Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48:63–72
Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P (2009) Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA 106:2035–2040. doi:10.1073/pnas.0811168106
Hua K, Oishi K, Zhang J et al (2009) Mapping of functional areas in the human cortex based on connectivity through association fibers. Cereb Cortex 19:1889–1895. doi:10.1093/cercor/bhn215
Hui KK, Liu J, Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N (2005) The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage 27:479–496
Irimia A, Goh SYM, Torgerson CM, Vespa PM, Van Horn JD (2014) Structural and connectomic neuroimaging for the personalized study of longitudinal alterations in cortical shape, thickness and connectivity after traumatic brain injury. J Neurosurg Sci 58(3):129–144
Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Jonsdottir J, Cattaneo D, Recalcati M, Regola A, Rabuffetti M, Ferrarin M, Casiraghi A (2010) Task-oriented biofeedback to improve gait in individuals with chronic stroke: motor learning approach. Neurorehabil Neural Repair 24(5):478–485
Kleiser R, Staempfli P, Valavanis A, Boesiger P, Kollias S (2010) Impact of fMRI-guided advanced DTI fiber tracking techniques on their clinical applications in patients with brain tumors. Neuroradiology 52:37–46
Lagana M, Rovaris M, Ceccarelli A, Venturelli C, Marini S, Baselli G (2010) DTI parameter optimisation for acquisition at 1.5 T: SNR analysis and clinical application. Comput Intell Neurosci 2010:8
Leemans A, Jones DK (2009) The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61:1336–1349
Levin HS (2003) Neuroplasticity following non-penetrating traumatic brain injury. Brain Inj 17:665–674
Makris N, Worth A, Papadimitriou G, Stakes J, Caviness V, Kennedy D, Pandya D, Kaplan E, Sorensen A, Wu O (1997) Morphometry of in vivo human white matter association pathways with diffusion-weighted magnetic resonance imaging. Ann Neurol 42:951–962
Makris N, Pandya DN, Normandin JJ, Papadimitriou GM, Rauch SL, Caviness VS, Kennedy DN (2002) Quantitative DT-MRI investigations of the human cingulum bundle. CNS Spectr 7:522–528
Makris N, Biederman J, Monuteaux MC, Seidman LJ (2009) Toward conceptualizing a neural system-based anatomy of attention-deficit/hyperactivity disorder. Dev Neurosci 31(1–2):36–49. doi:10.1159/000207492
Makris N, Preti M, Asami T, Pelavin P, Campbell B, Papadimitriou G, Kaiser J, Baselli G, Westin C, Shenton M (2013) Human middle longitudinal fascicle: variations in patterns of anatomical connections. Brain Struct Funct 218:951–968
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45:651–660
McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Dale AM, Halgren E (2008) Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology 71:1869–1876. doi:10.1212/01.wnl.0000327824.05348.3b
Morgan VL, Mishra A, Newton AT, Gore JC, Ding Z (2009) Integrating functional and diffusion magnetic resonance imaging for analysis of structure-function relationship in the human language network. PLoS One 4:e6660
Mori S, Oishi K, Faria AV (2009) White matter atlases based on diffusion tensor imaging. Curr Opin Neurol 22:362–369. doi:10.1097/WCO.0b013e32832d954b
Newton JM, Ward NS, Parker GJ, Deichmann R, Alexander DC, Friston KJ, Frackowiak RS (2006) Non-invasive mapping of corticofugal fibres from multiple motor areas—relevance to stroke recovery. Brain 129:1844–1858. doi:10.1093/brain/awl106
Paulesu E, Goldacre B, Scifo P, Cappa SF, Gilardi MC, Castiglioni I, Perani D, Fazio F (1997) Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. NeuroReport 8:2011–2016
Preti MG, Makris N, Papadimitriou G, Laganà MM, Griffanti L, Clerici M, Nemni R, Westin C, Baselli G, Baglio F (2014) A novel approach of groupwise fMRI-guided tractography allowing to characterize the clinical evolution of Alzheimer’s disease. PLoS One 9:e92026
Propper RE, O’Donnell LJ, Whalen S, Tie Y, Norton IH, Suarez RO, Zollei L, Radmanesh A, Golby AJ (2010) A combined fMRI and DTI examination of functional language lateralization and arcuate fasciculus structure: effects of degree versus direction of hand preference. Brain Cogn 73:85–92
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. doi:10.1073/pnas.98.2.676
Rehme AK, Grefkes C (2013) Cerebral network disorders after stroke: evidence from imaging-based connectivity analyses of active and resting brain states in humans. J Physiol 591(1):17–31. doi:10.1113/jphysiol.2012.243469
Reuter-Lorenz PA, Park DC (2014) How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol Rev 24:355–370
Rosso C, Valabregue R, Attal Y et al (2013) Contribution of corticospinal tract and functional connectivity in hand motor impairment after stroke. PLoS One 8:e73164. doi:10.1371/journal.pone.0073164
Schlösser RG, Nenadic I, Wagner G, Güllmar D, von Consbruch K, Köhler S, Schultz CC, Koch K, Fitzek C, Matthews PM (2007) White matter abnormalities and brain activation in schizophrenia: a combined DTI and fMRI study. Schizophr Res 89:1–11
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219
Staempfli P, Reischauer C, Jaermann T, Valavanis A, Kollias S, Boesiger P (2008) Combining fMRI and DTI: a framework for exploring the limits of fMRI-guided DTI fiber tracking and for verifying DTI-based fiber tractography results. Neuroimage 39:119–126
Tuch DS, Reese TG, Wiegell MR, Wedeen VJ (2003) Diffusion MRI of complex neural architecture. Neuron 40:885–895
Turken AU, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD (2008) Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage 42:1032–1044
van Eimeren L, Niogi SN, McCandliss BD, Holloway ID, Ansari D (2008) White matter microstructures underlying mathematical abilities in children. NeuroReport 19:1117–1121. doi:10.1097/WNR.0b013e328307f5c1
Vernooij M, Smits M, Wielopolski P, Houston G, Krestin G, van der Lugt A (2007) Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right-and left-handed healthy subjects: a combined fMRI and DTI study. Neuroimage 35:1064–1076
Wittenberg GF (2010) Experience, cortical remapping, and recovery in brain disease. Neurobiol Dis 37:252–258
Worsley KJ, Liao C, Aston J, Petre V, Duncan G, Morales F, Evans A (2002) A general statistical analysis for fMRI data. Neuroimage 15:1–15
Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S (2010) Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Exp Brain Res 204:57–70
Acknowledgments
This work was supported by 2014–2015 Ricerca Corrente (Italian Ministry of Health) (ES, MML, MGP, NB, PC, MC) and by the following funds: R21NS077059, R21NS079905, R01AG042512, R21EB016449 (NM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Rights and permissions
About this article
Cite this article
Scaccianoce, E., Laganà, M.M., Baglio, F. et al. Combined DTI–fMRI Analysis for a Quantitative Assessment of Connections Between WM Bundles and Their Peripheral Cortical Fields in Verbal Fluency. Brain Topogr 29, 814–823 (2016). https://doi.org/10.1007/s10548-016-0516-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-016-0516-0