Abstract
Background and aims
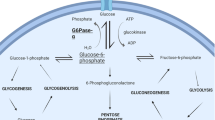
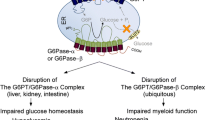
Glycogen storage disease type Ib (GSD1b) is a rare metabolic and immune disorder caused by a deficiency in the glucose-6-phosphate transporter (G6PT) and characterized by impaired glucose homeostasis, myeloid dysfunction, and long-term risk of hepatocellular adenomas. Despite maximal therapy, based on a strict diet and on granulocyte colony-stimulating factor treatment, long-term severe complications still develop. Understanding the pathophysiology of GSD1b is a prerequisite to develop new therapeutic strategies and depends on the availability of animal models. The G6PT-KO mouse mimics the human disease but is very fragile and rarely survives weaning. We generated a conditional G6PT-deficient mouse as an alternative model for studying the long-term pathophysiology of the disease. We utilized this conditional mouse to develop an inducible G6PT-KO model to allow temporally regulated G6PT deletion by the administration of tamoxifen (TM).
Methods
We generated a conditional G6PT-deficient mouse utilizing the CRElox strategy. Histology, histochemistry, and phenotype analyses were performed at different times after TM-induced G6PT inactivation. Neutrophils and monocytes were isolated and analyzed for functional activity with standard techniques.
Results
The G6PT-inducible KO mice display the expected disturbances of G6P metabolism and myeloid dysfunctions of the human disorder, even though with a milder intensity.
Conclusions
TM-induced inactivation of G6PT in these mice leads to a phenotype which mimics that of human GSD1b patients. The conditional mice we have generated represent an excellent tool to study the tissue-specific role of the G6PT gene and the mechanism of long-term complications in GSD1b.





Similar content being viewed by others
References
Bioulac-Sage P, Rebouissou S, Thomas C et al (2007) Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology 46:740–748
Chen YT, Cornblath M, Sidbury JB (1984) Cornstarch therapy in type I glycogen-storage disease. N Engl J Med 310:171–175
Chen LY, Shieh JJ, Lin B et al (2003) Impaired glucose homeostasis, neutrophil trafficking and function in mice lacking the glucose-6-phosphate transporter. Hum Mol Genet 12:2547–2558
Cheng L, Guo J, Sun L et al (2010) Postnatal tissue-specific disruption of transcription factor FoxN1 triggers acute thymic atrophy. J Biol Chem 285:5836–5847
Chou JY, Matern D, Mansfield BC et al (2002) Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr Mol Med 2:121–143
Chou JY, Jun HS, Mansfield BC (2010) Glycogen storage disease type I and G6Pase-beta deficiency: etiology and therapy. Nat Rev Endocrinol 6:676–688
Danielian PS, White R, Hoare SA et al (1993) Identification of residues in the estrogen receptor that confer differential sensitivity to estrogen and hydroxytamoxifen. Mol Endocrinol 7:232–240
Dick J, Kumar N, Horsfield C et al (2012) AA amyloidosis in a patient with glycogen storage disorder and progressive chronic kidney disease. Clin Kidney J 5:559–561
Dieckgraefe BK, Korzenik JR, Husain A et al (2002) Association of glycogen storage disease 1b and Crohn disease: results of a north American survey. Eur J Pediatr 161(Suppl 1):S88–S92
Fawell SE, White R, Hoare S et al (1990) Inhibition of estrogen receptor-DNA binding by the “pure” antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci U S A 87:6883–6887
Gitzelmann R, Bosshard NU (1993) Defective neutrophil and monocyte functions in glycogen storage disease type Ib: a literature review. Eur J Pediatr 152(Suppl 1):S33–S38
Greene HL, Slonim AE, O’Neill JA Jr et al (1976) Continuous nocturnal intragastric feeding for management of type 1 glycogen-storage disease. N Engl J Med 294:423–425
Hayashi S, McMahon AP (2002) Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244:305–318
Jun HS, Cheung YY, Lee YM et al (2012) Glucose-6-phosphatase-beta, implicated in a congenital neutropenia syndrome, is essential for macrophage energy homeostasis and functionality. Blood 119:4047–4055
Kilpatrick L, Garty BZ, Lundquist KF et al (1990) Impaired metabolic function and signaling defects in phagocytic cells in glycogen storage disease type 1b. J Clin Invest 86:196–202
Kuijpers TW, Maianski NA, Tool AT et al (2003) Apoptotic neutrophils in the circulation of patients with glycogen storage disease type 1b (GSD1b). Blood 101:5021–5024
Littlewood TD, Hancock DC, Danielian PS et al (1995) A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res 23:1686–1690
McCawley LJ, Korchak HM, Cutilli JR et al (1993) Interferon-gamma corrects the respiratory burst defect in vitro in monocyte-derived macrophages from glycogen storage disease type 1b patients. Pediatr Res 34:265–269
Sun MS, Pan CJ, Shieh JJ et al (2002) Sustained hepatic and renal glucose-6-phosphatase expression corrects glycogen storage disease type Ia in mice. Hum Mol Genet 11:2155–2164
Visser G, Rake JP, Fernandes J et al (2000) Neutropenia, neutrophil dysfunction, and inflammatory bowel disease in glycogen storage disease type Ib: results of the European Study on Glycogen Storage Disease type I. J Pediatr 137:187–191
Visser G, Rake JP, Labrune P et al (2002) Granulocyte colony-stimulating factor in glycogen storage disease type 1b. Results of the European Study on Glycogen Storage Disease type 1. Eur J Pediatr 161(Suppl 1):S83–S87
Visser G, de Jager W, Verhagen LP et al (2012) Survival, but not maturation, is affected in neutrophil progenitors from GSD-1b patients. J Inherit Metab Dis 35:287–300
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Federica Raggi, Anna Livia Pissavino, Roberta Resaz, Daniela Segalerba, Andrea Puglisi, Cristina Vanni, Francesca Antonini, Genny Del Zotto, Alessandra Gamberucci, Paola Marcolongo, Maria Carla Bosco, Federica Grillo, Luca Mastracci, and Alessandra Eva declare that they have no conflict of interest.
Financial support
This work was supported by grants from Associazione Italiana Glicogenosi (to A.E.), from Compagnia di San Paolo (grant 318 to A.E.), and Ministero della Salute.
Additional information
Communicated by: Jörn Oliver Sass
Electronic supplementary material
Below are the links to the electronic supplementary material.
ESM 1
(DOCX 29 kb)
ESM 2
Figure 1. Analysis of G6PT deletion in TM-G6PT−/− mice. The extent of deletion of G6PT exons in TM-G6PT−/− mice was evaluated by PCR on the DNA extracted from several tissues. Evaluation of G6PT excision on DNA from the tails (T1, T2, and T3) of three TM-G6PT−/− mice using (a) the primer pair EX5S and EX6AS or (b) the primer pair 1S and EX6AS. PCR analysis with primer pair EX5S and EX6AS of DNA from the (c) liver and kidney and (d) spleen and BM of TM-G6PT−/− mice. PCR analysis with primer pair 1S and EX6AS of DNA from the (e) kidney and liver and (f) spleen and BM of TM-G6PT−/− mice. The results of PCR analysis of DNA from tissues of TM-treated G6PT+/+ are shown as the control. MW, molecular weight markers. (PNG 842 kb)
ESM 3
Figure 2. Phenotype analysis in TM-G6PT−/− mice. a Liver and kidney weights (expressed as percentage of the body weight) in TM-G6PT−/− mice were compared to those of wt mice. Time curves of (b) blood glucose and (c) lactate in response to 24 h of fasting. d Serum uric acid level of TM-G6PT−/− mice was determined at the indicated times, days (d) or months (m), following TM treatment. Five TM-G6PT−/− mice were analyzed for each group. Because similar levels of the various metabolites were observed in each group of control mice, the results shown are pooled data of mice aged 2–8 months. Data are presented as mean ±SD. Values that are significantly different from control mice (*p < 0.05) are indicated. (PNG 151 kb)
ESM 4
Figure 3. Influx of G6P, glucose, and phosphate into mouse liver microsomal vesicles. The permeability of G6P (green trace in all panels) was tested compared to a poor permeant, such as sucrose (red trace in all panels), which causes a prolonged shrinking of vesicles, revealed by the increase in light scattering, permeant compounds, such as glucose (black trace in all panels), and PI (blue trace in all panels). These compounds cause a transient shrinking, followed by a swelling phase (decrease in light scattering), whose rate parallels the entry of these compounds into the vesicles. Addition of the detergent Triton X-100 to the system resulted in a fast decrease of the signal to a comparable basal value (not shown), reflecting the complete permeabilization of the vesicles. (PNG 287 kb)
ESM 5
Figure 4. Histological appearance of the intestine in adult TM-G6PT−/− mice. Five-month-old G6PT+/+ (a) and TM-G6PT−/− (b and c) mice were stained with H&E. The villous axis of the small intestine of TM-G6PT−/− mice are packed with globoid cells filled with amorphous material deposition. The material stained positive with Congo red and displayed green birefringence when viewed under polarized light (data not shown), confirming amyloid deposition. a, b 10× magnification; c 40× magnification. (PNG 2025 kb)
ESM 6
Figure 5. Immunoclassification of adenoma in a TM-G6PT−/− mouse. The liver was fixed with 10% formalin and paraffin embedded. Serial sections were stained with H&E or treated with anti-serum amyloid (SAA) or anti-glutamine synthetase (GS) antibody. a, b H&E-stained sections of the nodule showing little atypia and no portal tracts. c, d The nodule shows strong positivity for GS within the lesion and (e, f) faint but positive staining for SAA. The surrounding non-lesional parenchyma shows normal zonation of the expression of GS in perivascular hepatocytes and intense positivity of SAA in amyloid deposits. a, c, e 2× magnification; b, d, f 20× magnification. (PNG 4239 kb)
ESM 7
Figure 6. Evaluation of neutrophil cell number and G-CSF secretion. a Total cell number in combined BM aspirates of G6PT+/+ and TM-G6PT−/− mice femur and tibia bones. The data are presented as the mean ± SD of six different experiments. b BM neutrophil number. Neutrophils were purified from 10 × 106 BM total cells. The data are presented as the mean ± SD of six different experiments. c Peritoneal neutrophil number in G6PT+/+ and TM-G6PT−/− mice. The data are presented as the mean ± SD of six different experiments. p-Values of TM-G6PT−/− relative to G6PT+/+ mice: *p ≤ 0.05. d The serum G-CSF concentration in six TM-G6PT−/− and six G6PT+/+ mice was measured by ELISA. The results are shown as a box plot and expressed as ng/mL. The boxes contain the values falling between the 25th and 75th percentiles, the horizontal lines represent mean values, and the whiskers (lines that extend from the boxes) represent the highest and lowest values for each group. (PNG 47 kb)
Rights and permissions
About this article
Cite this article
Raggi, F., Pissavino, A.L., Resaz, R. et al. Development and characterization of an inducible mouse model for glycogen storage disease type Ib. J Inherit Metab Dis 41, 1015–1025 (2018). https://doi.org/10.1007/s10545-018-0211-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-018-0211-2