Abstract
Introduction
McArdle disease is an inborn disorder of muscle glycogen metabolism that produces exercise intolerance, and has been recently associated with low values of lean mass (LM) and bone mineral content (BMC) and density (BMD) in affected adults. Here we aimed to study whether this bone health problem begins in childhood.
Methods
Forty children and adolescents were evaluated: 10 McArdle disease and 30 control children (mean age of both groups, 13 ± 2y). Body composition was evaluated by dual-energy X-ray absorptiometry and creatine kinase (CK) levels were determined in the patients as an estimate of muscle damage.
Results
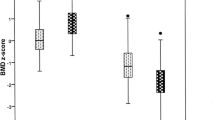
Legs bone mass was significantly lower in patients than in controls (−36% for BMC and −22% for BMD). Moreover, patients had significantly higher LM values in the legs than controls, whereas no difference was found for fat mass. CK levels were positively associated with LM in McArdle patients. A correlation was found between LM and BMD variables in the control group but not in McArdle patients.
Conclusion
We have identified a ‘non-osteogenic muscle hypertrophy’ in children with McArdle disease. This phenomenon warrants special attention since low osteogenesis at an early age predicts a high risk for osteoporosis later in life.
Similar content being viewed by others
References
Bachrach LK (2014) Diagnosis and treatment of pediatric osteoporosis. Curr Opin Endocrinol Diabet Obes 21(6):454–460
Bertoldo F, Zappini F, Brigo M et al (2015) Prevalence of asymptomatic vertebral fractures in late-onset Pompe disease. J Clin Endocrinol Metab 100(2):401–406. https://doi.org/10.1210/jc.2014-2763
Demirsoy U, Sarper N, Gelen SA, Zengin E, Kum T, Demir H (2017) The association of oral vitamin D and calcium supplementation with bone mineral density in pediatric acute lymphoblastic leukemia patients. J Pediatr Hematol Oncol 39(4):287–292
Evans WJ, Cannon JG (1991) 3 the metabolic effects of exercise-induced muscle damage. Exerc Sport Sci Rev 19(1):99–126
Garcia-Benitez S, Fleck SJ, Naclerio F, Martin MA, Lucia A (2013) Resistance (weight lifting) training in an adolescent with McArdle disease. J Child Neurol 28(6):805–808. https://doi.org/10.1177/0883073812451328
Golden NH, Abrams SA (2014) Optimizing bone health in children and adolescents. Pediatrics 134(4):e1229–e1243
Komulainen J, Kalliokoski R, Koskinen S, Drost M, Kuipers H, Hesselink M (2000) Controlled lengthening or shortening contraction-induced damage is followed by fiber hypertrophy in rat skeletal muscle. Int J Sports Med 21(02):107–112
Lucia A, Ruiz JR, Santalla A et al (2012) Genotypic and phenotypic features of McArdle disease: insights from the Spanish national registry. J Neurol Neurosurg Psychiatry 83(3):322–328. https://doi.org/10.1136/jnnp-2011-301593
Marrani E, Giani T, Simonini G, Cimaz R (2017) Pediatric osteoporosis: diagnosis and treatment considerations. Drugs 77(6):679-695. https://doi.org/10.1007/s40265-017-0715-3
Melis D, Rossi A, Pivonello R et al (2016) Reduced bone mineral density in glycogen storage disease type III: evidence for a possible connection between metabolic imbalance and bone homeostasis. Bone 86:79–85. https://doi.org/10.1016/j.bone.2016.02.012
Mitchell JA, Chesi A, Elci O et al (2015) Genetics of bone mass in childhood and adolescence: effects of sex and maturation interactions. J Bone Miner Res 30(9):1676–1683
Nogales-Gadea G, Santalla A, Ballester-Lopez A et al (2016) Exercise and preexercise nutrition as treatment for McArdle disease. Med Sci Sports Exerc 48(4):673–679. https://doi.org/10.1249/MSS.0000000000000812
Noori N, Kovesdy CP, Bross R et al (2011) Novel equations to estimate lean body mass in maintenance hemodialysis patients. Am J Kidney Dis 57(1):130–139
Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans RO, Bakker SJ (2009) Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis 207(2):534–540
Patel SS, Molnar MZ, Tayek JA et al (2013) Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle 4(1):19–29
Perez M, Mate-Munoz JL, Foster C et al (2007) Exercise capacity in a child with McArdle disease. J Child Neurol 22(7):880–882. https://doi.org/10.1177/0883073807304206
Rake JP, Visser G, Huismans D et al (2003) Bone mineral density in children, adolescents and adults with glycogen storage disease type Ia: a cross-sectional and longitudinal study. J Inherit Metab Dis 26(4):371–384
Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R (2004) The ‘muscle-bone unit’during the pubertal growth spurt. Bone 34(5):771–775
Rizzoli R, Bianchi ML, Garabédian M, McKay HA, Moreno LA (2010) Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone 46(2):294–305
Rodríguez-Gómez I, Santalla A, Diez-Bermejo J et al (2018) A new condition in McArdle disease: poor bone health—benefits of an active lifestyle. Med Sci Sports Exerc 50(1):3-10. https://doi.org/10.1249/MSS.0000000000001414
Santalla A, Munguía-Izquierdo D, Brea-Alejo L et al (2014a) Feasibility of resistance training in adult McArdle patients: clinical outcomes and muscle strength and mass benefits. Front Aging Neurosci 6:334
Santalla A, Nogales-Gadea G, Ortenblad N et al (2014b) McArdle disease: a unique study model in sports medicine. Sports Med 44(11):1531–1544. https://doi.org/10.1007/s40279-014-0223-5
Santalla A, Nogales-Gadea G, Encinar AB et al (2017) Genotypic and phenotypic features of all Spanish patients with McArdle disease: a 2016 update. BMC Genomics 18(8):819
Scalco RS, Morrow JM, Booth S, Chatfield S, Godfrey R, Quinlivan R (2017) Misdiagnosis is an important factor for diagnostic delay in McArdle disease. Neuromuscular Disord 27(9):852-855. https://doi.org/10.1016/j.nmd.2017.04.013
Schoenau E (2005) From mechanostat theory to development of the" functional muscle-bone-unit". J Musculoskelet Nueronal Interact 5(3):232
Schoenau E, Frost H (2002) The" muscle-bone unit" in children and adolescents. Calcif Tissue Int 70(5):405–407
Schonau E, Schwahn B, Rauch F (2002) The muscle-bone relationship: methods and management — perspectives in glycogen storage disease. Eur J Pediatr 161(Suppl 1):S50–S52. https://doi.org/10.1007/s00431-002-1003-z
Schwahn B, Rauch F, Wendel U, Schonau E (2002) Low bone mass in glycogen storage disease type 1 is associated with reduced muscle force and poor metabolic control. J Pediatr 141(3):350–356. https://doi.org/10.1067/mpd.2002.126456
Smith TJ, Tripkovic L, Lanham-New SA, Hart KH (2017) Vitamin D in adolescence: evidence-based dietary requirements and implications for public health policy. Proc Nutri Soc 1–10
Vicente-Rodríguez G (2006) How does exercise affect bone development during growth? Sports Med 36(7):561–569
Vicente-Rodriguez G, Ara I, Pérez-Gómez J, Dorado C, Calbet JA (2005) Muscular development and physical activity as major determinants of femoral bone mass acquisition during growth. Br J Sports Med 39(9):611–616
Wernig A, Irintchev A, Weisshaupt P (1990) Muscle injury, cross-sectional area and fibre type distribution in mouse soleus after intermittent wheel-running. J Physiol 428(1):639–652
Acknowledgments
This study was funded by the Cátedra Real Madrid - Universidad Europea de Madrid (P2016/RM25), Fondo de Investigaciones Sanitarias (A.L., PI15/00558; G.N.G, PI15/01756 and CP14/00032, J.A. PI14/00903), AFM Telethon Trampoline Grant #21108, the Biomedical Research Networking Center on Frailty and Healthy Aging (CIBERFES) and FEDER funds from the European Union (CB16/10/00477). Irene Rodríguez Gómez has received a PhD grant from the Universidad de Castilla-La Mancha “Contratos predoctorales para la formación de personal investigador en el marco del Plan Propio de I + D + i, cofinanciados por el Fondo Social Europeo” (2014/10340).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Communicated by: Bridget Wilcken
Electronic supplementary material
ESM 1
(DOC 36 kb)
Rights and permissions
About this article
Cite this article
Rodríguez-Gómez, I., Santalla, A., Díez-Bermejo, J. et al. Non-osteogenic muscle hypertrophy in children with McArdle disease. J Inherit Metab Dis 41, 1037–1042 (2018). https://doi.org/10.1007/s10545-018-0170-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-018-0170-7