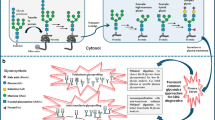
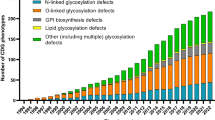
Abstract
Pattern recognition, using a group of characteristic, or discriminating features, is a powerful tool in metabolic diagnostic. A classic example of this approach is used in biochemical analysis of urine organic acid analysis, where the reporting depends more on the correlation of pertinent positive and negative findings, rather than on the absolute values of specific markers. Similar uses of pattern recognition in the field of biochemical genetics include the interpretation of data obtained by metabolomics, like glycomics, where a recognizable pattern or the presence of a specific glycan sub-fraction can lead to the direct diagnosis of certain types of congenital disorders of glycosylation. Another indispensable tool is the use of clinical pattern recognition–or syndromology–relying on careful phenotyping. While genomics might uncover variants not essential in the final clinical expression of disease, and metabolomics could point to a mixture of primary but also secondary changes in biochemical pathways, phenomics describes the clinically relevant manifestations and the full expression of the disease. In the current review we apply phenomics to the field of congenital disorders of glycosylation, focusing on recognizable differentiating findings in glycosylation disorders, characteristic dysmorphic features and malformations in PMM2-CDG, and overlapping patterns among the currently known glycosylation disorders based on their pathophysiological basis.


Similar content being viewed by others
References
Al Teneiji A, Bruun TUJ, Sidky S et al (2017) Phenotypic and genotypic spectrum of congenital disorders of glycosylation type I and type II. Mol Genet Metab 120:235–242
Al-Maawali AA, Miller E, Schulze A et al (2014) Subcutaneous fat pads on body MRI--an early sign of congenital disorder of glycosylation PMM2-CDG (CDG1a). Pediatr Radiol 44:222–225
Antoun H, Villeneuve N, Gelot A et al (1999) Cerebellar atrophy: an important feature of carbohydrate deficient glycoprotein syndrome type 1. Pediatr Radiol 29:194–198
Artigas J, Cardo E, Pineda M et al (1998) Phosphomannomutase deficiency and normal pubertal development. J Inherit Metab Dis 21:78–79
Barone R, Carrozzi M, Parini R et al (2015) A nationwide survey of PMM2-CDG in Italy: high frequency of a mild neurological variant associated with the L32R mutation. J Neurol 262:154–164
Barone R, Sturiale L, Sofia V et al (2008) Clinical phenotype correlates to glycoprotein phenotype in a sib pair with CDG-Ia. Am J Med Genet A 146A:2103–2108
Bengtson P, Ng BG, Jaeken J et al (2016) Serum transferrin carrying the xeno-tetrasaccharide NeuAc-gal-GlcNAc2 is a biomarker of ALG1-CDG. J Inherit Metab Dis 39(1):107–114
Bortot B, Cosentini D, Faletra F et al (2013) PMM2-CDG: phenotype and genotype in four affected family members. Gene 531:506–509
Briones P, Vilaseca MA, Schollen E et al (2002) Biochemical and molecular studies in 26 Spanish patients with congenital disorder of glycosylation type Ia. J Inherit Metab Dis 25:635–646
Brum JM, De Rizzo IMPO, de Mello WD, Speck-Martins CE (2008) Congenital disorder of glycosylation type Ia: a non-progressive encephalopathy associated with multisystemic involvement. Arq Neuropsiquiatr 66:545–548
Bubel S, Peters V, Klein C et al (2002) CDG (congenital disorders of glycosylation). Differential hereditary ataxia in adulthood diagnosis. Nervenarzt 73:754–760
Buczkowska A, Swiezewska E, Lefeber DJ (2015) Genetic defects in dolichol metabolism. J Inherit Metab Dis 38(1):157–169
Clayton PT, Winchester BG, Keir G (1992) Hypertrophic obstructive cardiomyopathy in a neonate with the carbohydrate-deficient glycoprotein syndrome. J Inherit Metab Dis 15:857–861
Cossins J, Belaya K, Hicks D et al (2013) Congenital myasthenic syndromes due to mutations in ALG2 and ALG14. Brain J Neurol 136:944–956
de Lonlay P, Seta N, Barrot S et al (2001) A broad spectrum of clinical presentations in congenital disorders of glycosylation I: a series of 26 cases. J Med Genet 38:14–19
de Michelena MI, Franchi LM, Summers PG et al (1999) Carbohydrate-deficient glycoprotein syndrome due to phosphomannomutase deficiency: the first reported cases from Latin America. Am J Med Genet 84:481–483
Dörre K, Olczak M, Wada Y et al (2015) A new case of UDP-galactose transporter deficiency (SLC35A2-CDG): molecular basis, clinical phenotype, and therapeutic approach. J Inherit Metab Dis 38(5):931–940
Edwards M, McKenzie F, O’callaghan S et al (2006) Prenatal diagnosis of congenital disorder of glycosylation type Ia (CDG-Ia) by cordocentesis and transferrin isoelectric focussing of serum of a 27-week fetus with non-immune hydrops. Prenat Diagn 26:985–988
Endo T (2015) Glycobiology of α-dystroglycan and muscular dystrophy. J Biochem 157(1):1–12
Enns GM, Steiner RD, Buist N et al (2002) Clinical and molecular features of congenital disorder of glycosylation in patients with type 1 sialotransferrin pattern and diverse ethnic origins. J Pediatr 141:695–700
Erlandson A, Bjursell C, Stibler H et al (2001) Scandinavian CDG-Ia patients: genotype/phenotype correlation and geographic origin of founder mutations. Hum Genet 108:359–367
Garel C, Baumann C, Besnard M et al (1998) Carbohydrate-deficient glycoprotein syndrome type I: a new cause of dysostosis multiplex. Skelet Radiol 27:43–45
Grünewald S, Schollen E, Van Schaftingen E et al (2001) High residual activity of PMM2 in patients’ fibroblasts: possible pitfall in the diagnosis of CDG-Ia (phosphomannomutase deficiency). Am J Hum Genet 68:347–354
Harding BN, Dunger DB, Grant DB, Erdohazi M (1988) Familial olivopontocerebellar atrophy with neonatal onset: a recessively inherited syndrome with systemic and biochemical abnormalities. J Neurol Neurosurg Psychiatry 51:385–390
Hertz-Pannier L, Déchaux M, Sinico M et al (2006) Congenital disorders of glycosylation type I: a rare but new cause of hyperechoic kidneys in infants and children due to early microcystic changes. Pediatr Radiol 36(2):108–114
Höck M, Wegleiter K, Ralser E et al (2015) ALG8-CDG: novel patients and review of the literature. Orphanet J Rare Dis 10:73
Honzík T, Magner M, Krijt J et al (2012) Clinical picture of S-adenosylhomocysteine hydrolase deficiency resembles phosphomannomutase 2 deficiency. Mol Genet Metab 107:611–613
Imtiaz F, Worthington V, Champion M et al (2000) Genotypes and phenotypes of patients in the UK with carbohydrate-deficient glycoprotein syndrome type 1. J Inherit Metab Dis 23:162–174
Işıkay S, Başpınar O, Yılmaz K (2014) A case of congenital disorder of glycosylation ia presented with recurrent pericardial effusion. Iran J Pediatr 24:652–654
Jaeken J, Péanne R (2017) What is new in CDG? J Inherit Metab Dis 40(4):569–586
Kasapkara ÇS, Barış Z, Kılıç M et al (2017) PMM2-CDG and sensorineural hearing loss. J Inherit Metab Dis 40:629-630
Kjaergaard S, Schwartz M, Skovby F (2001) Congenital disorder of glycosylation type Ia (CDG-Ia): phenotypic spectrum of the R141H/F119L genotype. Arch Dis Child 85:236–239
Krasnewich DM, Holt GD, Brantly M et al (1995) Abnormal synthesis of dolichol-linked oligosaccharides in carbohydrate-deficient glycoprotein syndrome. Glycobiology 5(5):503–510
Laplace O, Voegtle R, Rigolet M-H et al (2003) Early ocular manifestations in an infant with carbohydrate-deficient glycoprotein syndrome type Ia. J Pediatr Ophthalmol Strabismus 40:179–181
Léticée N, Bessières-Grattagliano B, Dupré T et al (2010) Should PMM2-deficiency (CDG Ia) be searched in every case of unexplained hydrops fetalis? Mol Genet Metab 101:253–257
Lumaka A, Cosemans N, Lulebo Mampasi A et al (2017) Facial dysmorphism is influenced by ethnic background of the patient and of the evaluator. Clin Genet 92:166–171
Marques-da-Silva D, Dos Reis FV, Monticelli M et al (2017a) Liver involvement in congenital disorders of glycosylation (CDG). A systematic review of the literature. J Inherit Metab Dis 40(2):195–207
Marques-da-Silva D, Francisco R, Webster D et al (2017b) Cardiac complications of congenital disorders of glycosylation (CDG): a systematic review of the literature. J Inherit Metab Dis 40(5):657–672
Monin M-L, Mignot C, De Lonlay P et al (2014) 29 French adult patients with PMM2-congenital disorder of glycosylation: outcome of the classical pediatric phenotype and depiction of a late-onset phenotype. Orphanet J Rare Dis 9:207
Morava E, Cser B, Kárteszi J et al (2004) Screening for CDG type Ia in Joubert syndrome. Med Sci Monit Int Med J Exp Clin Res 10:CR469–CR472
Morava E, Tiemes V, Thiel C et al (2016) ALG6-CDG: a recognizable phenotype with epilepsy, proximal muscle weakness, ataxia and behavioral and limb anomalies. J Inherit Metab Dis 39:713–723
Noelle V, Knuepfer M, Pulzer F et al (2005) Unusual presentation of congenital disorder of glycosylation type 1a: congenital persistent thrombocytopenia, hypertrophic cardiomyopathy and hydrops-like aspect due to marked peripheral oedema. Eur J Pediatr 164:223–226
Panneerselvam K, Freeze HH (1996) Mannose corrects altered N-glycosylation in carbohydrate-deficient glycoprotein syndrome fibroblasts. J Clin invest. 97:1478-87
Pavone L, Fiumara A, Barone R et al (1996) Olivopontocerebellar atrophy leading to recognition of carbohydrate-deficient glycoprotein syndrome type I. J Neurol 243:700–705
Pérez B, Briones P, Quelhas D et al (2011) The molecular landscape of phosphomannose mutase deficiency in iberian peninsula: identification of 15 population-specific mutations. JIMD Rep 1:117–123
Regal L, van Hasselt PM, Foulquier F et al (2015) ALG11-CDG: three novel mutations and further characterization of the phenotype. Mol Genet Metab Rep 2:16–19
Resende C, Carvalho C, Alegria A et al (2014) Congenital disorders of glycosylation with neonatal presentation. BMJ Case Rep
Riley LG, Cowley MJ, Gayevskiy V et al (2017) A SLC39A8 variant causes manganese deficiency, and glycosylation and mitochondrial disorders. J Inherit Metab Dis 40(2):261–269
Rind N, Schmeiser V, Thiel C et al (2010) A severe human metabolic disease caused by deficiency of the endoplasmatic mannosyltransferase hALG11 leads to congenital disorder of glycosylation-Ip. Hum Mol Genet 19:1413–1424
Romano S, Bajolle F, Valayannopoulos V et al (2009) Conotruncal heart defects in three patients with congenital disorder of glycosylation type Ia (CDG Ia). J Med Genet 46:287–288
Rudaks LI, Andersen C, Khong TY et al (2012) Hypertrophic cardiomyopathy with cardiac rupture and tamponade caused by congenital disorder of glycosylation type Ia. Pediatr Cardiol 33:827–830
Senderek J, Müller JS, Dusl M et al (2011) Hexosamine biosynthetic pathway mutations cause neuromuscular transmission defect. Am J Hum Genet 88:162–172
Serrano M, de Diego V, Muchart J et al (2015) Phosphomannomutase deficiency (PMM2-CDG): ataxia and cerebellar assessment. Orphanet J Rare Dis 10:138
Stark KL, Gibson JB, Hertle RW, Brodsky MC (2000) Ocular motor signs in an infant with carbohydrate-deficient glycoprotein syndrome type Ia. Am J Ophthalmol 130:533–535
Schiff M, Roda C, Monin ML et al (2017) Clinical, laboratory and molecular findings and long-term follow-up data in 96 French patients with PMM2-CDG (phosphomannomutase 2-congenital disorder of glycosylation) and review of the literature. J Med Genet 54(12):843–851
Tayebi N, Andrews DQ, Park JK et al (2002) A deletion-insertion mutation in the phosphomannomutase 2 gene in an African American patient with congenital disorders of glycosylation-Ia. Am J Med Genet 108:241–246
Tham E, Eklund EA, Hammarsjö A et al (2016) A novel phenotype in N-glycosylation disorders: Gillessen-Kaesbach-Nishimura skeletal dysplasia due to pathogenic variants in ALG9. Eur J Hum Genet EJHG 24:198–207
Thompson DA, Lyons RJ, Russell-Eggitt I et al (2013) Retinal characteristics of the congenital disorder of glycosylation PMM2-CDG. J Inherit Metab Dis 36:1039–1047
Thong MK, Fietz M, Nicholls C et al (2009) Congenital disorder of glycosylation type Ia in a Malaysian family: clinical outcome and description of a novel PMM2 mutation. J Inherit Metab Dis 32(Suppl 1):S41–S44
Truin G, Guillard M, Lefeber DJ et al (2008) Pericardial and abdominal fluid accumulation in congenital disorder of glycosylation type Ia. Mol Genet Metab 94:481–484
Vabres P, Sevin C, Amoric JC et al (1998) Skin manifestations of protein glycosylation deficiency, the CDG (carbohydrate deficient glycoprotein) type 1 syndrome. Ann Dermatol Venereol 125:715–716
van de Kamp JM, Lefeber DJ, Ruijter GJG et al (2007) Congenital disorder of glycosylation type Ia presenting with hydrops fetalis. J Med Genet 44:277–280
Veneselli E, Biancheri R, Di Rocco M, Tortorelli S (1998) Neurophysiological findings in a case of carbohydrate-deficient glycoprotein (CDG) syndrome type I with phosphomannomutase deficiency. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc 2:239–244
Vermeer S, Kremer HPH, Leijten QH et al (2007) Cerebellar ataxia and congenital disorder of glycosylation Ia (CDG-Ia) with normal routine CDG screening. J Neurol 254:1356–1358
Vleugels W, Haeuptle MA, Ng BG et al (2009a) RFT1 deficiency in three novel CDG patients. Hum Mutat 30:1428–1434
Vleugels W, Keldermans L, Jaeken J et al (2009b) Quality control of glycoproteins bearing truncated glycans in an ALG9-defective (CDG-IL) patient. Glycobiology 19(8):910–917
Wolthuis DFGJ, van Asbeck EV, Kozicz T, Morava E (2013) Abnormal fat distribution in PMM2-CDG. Mol Genet Metab 110:411–413
Wu X, Rush JS, Karaoglu D et al (2003) Deficiency of UDP-GlcNAc:dolichol phosphate N-Acetylglucosamine-1 phosphate transferase (DPAGT1) causes a novel congenital disorder of glycosylation type Ij. Hum Mutat 22:144–150
Acknowledgements
This study is supported by the 1805218 N FWO subsidie, in part by the Hayward Foundation and by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. Additionally this work was supported by the European Union’s Horizon 2020 research and innovation program under the ERA-NET Cofund action N° 643578 –EURO-CDG-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
C. Ferreira, R. Altassan, R. Francesco, D. Marquez-Da-Silva, J. Jaeken and E. Morava declare that they have no conflict of interest.
Additional information
Responsible Editor: Marc Patterson
Electronic supplementary material
ESM 1
(XLSX 47 kb)
Rights and permissions
About this article
Cite this article
Ferreira, C.R., Altassan, R., Marques-Da-Silva, D. et al. Recognizable phenotypes in CDG. J Inherit Metab Dis 41, 541–553 (2018). https://doi.org/10.1007/s10545-018-0156-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-018-0156-5