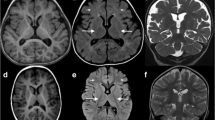
Abstract
Congenital disorders of glycosylation (CDG) are a group of hereditary metabolic diseases characterized by abnormal glycosylation of proteins and lipids. Often, multisystem disorders with central nervous system involvement and a large variety of clinical symptoms occur. The main characteristics are developmental delay, seizures, and ataxia. In this paper we report the clinical and biochemical characteristics of a 5-year-old girl with a defective galactosylation of N-glycans, resulting in developmental delay, muscular hypotonia, epileptic seizures, inverted nipples, and visual impairment. Next generation sequencing revealed a de novo mutation (c.797G > T, p.G266V) in the X-chromosomal gene SLC35A2 (solute carrier family 35, UDP-galactose transporter, member A2; MIM 300896). While this mutation was found heterozygous, random X-inactivation of the normal allele will lead to loss of normal SLC35A2 activity in respective cells. The functional relevance of the mutation was demonstrated by complementation of UGT-deficient MDCK-RCAr and CHO-Lec8 cells by normal UGT-expression construct but not by the mutant version. The effect of dietary galactose supplementation on glycosylation was investigated, showing a nearly complete normalization of transferrin glycosylation.



Similar content being viewed by others
Abbreviations
- ACTH:
-
Adrenocorticotropic hormone
- CDG:
-
Congenital disorder of glycosylation
- DPBS:
-
Dulbecco’s phosphate-buffered saline
- EEG:
-
Electroencephalography
- EOEE:
-
Early onset epileptic encephalopathy
- ER:
-
Endoplasmic reticulum
- ESI:
-
Electrospray ionization
- HPLC:
-
High performance liquid chromatography
- IEF:
-
Isoelectric focusing
- IMPP:
-
Immunoprecipitation
- MALDI:
-
Matrix-assisted laser desorption/ionization
- MRI:
-
Magnetic resonance imaging
- NSTs:
-
Nucleotide sugar transporters
- OAE:
-
Otoacoustic emissions
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SLC family:
-
Solute carrier family
- TF:
-
Transferrin
- UGT:
-
UDP-galactose transporter
References
Biffi S, Tamaro G, Bortot B, Zamberlan S, Severini GM, Carrozzi M (2007) Carbohydrate deficient transferrin (CDT) as a biochemical tool for the screening of congenital disorders of glycosylation (CDGs). Clin Biochem 40:1431–1434
Biskup S (2010) Molekualrgenetische und zytogenetische Diagnostik. Hochdurchsatz-Sequenzierung in der Humangenetischen Diagnostik. Next-generation sequencing in genetic diagnostics. J Lab Med 34(6):305–309
Clayton P, Winchester B, Di Tomaso E, Young E, Keir G, Rodeck C (1993) Carbohydrate-deficient glycoprotein syndrome: normal glycosylation in the fetus. Lancet 341(8850):956
Ferrari MC, Parini R, Di Rocco MD, Radetti G, Beck-Peccoz P, Persani L (2001) Lectin analyses of glycoprotein hormones in patients with congenital disorders of glycosylation. Eur J Endocrinol 144(4):409–416
Freeze HH (2013) Understanding human glycosylation disorders: biochemistry leads the charge. J Biol Chem 288(10):6936–6945
Hanßke B, Thiel C, Lübke T et al (2002) Deficiency of UDP-galactose: N-acetylglucosamine ß-1,4-galactosyltransferase I causes the congenital disorder of glycosylation type IId. J Clin Invest 109(6):725–733
Hiraoka S, Furuichi T, Nishimura G et al (2007) Nucleotide-sugar transporter SLC35D1 is critical to chondroitin sulfate synthesis in cartilage and skeletal development in mouse and human. Nat Med 13(11):1363–1367
Kabuß R, Ashikov A, Oelmann S, Gerardy-Schahn R, Bakker H (2005) Endoplasmic reticulum retention of the large splice variant of the UDP-galactose transporter is caused by a dilysine motif. Glycobiology 15(10):905–911
Kniffin CL, Hamosh A, Converse PJ, McKusick VA (2013) Solute carrier family 35 (UDP-GALACTOSE TRANSPORTER), MEMBER 2; SLC35A2. http://omim.org/entry/314375
Kodera H, Nakamura K, Osaka H et al (2013) De novo mutations in SLC35A2 encoding a UDP-galactose transporter cause early-onset epileptic encephalopathy. Humu 0272
Liu L, Xu YX, Hirschberg CB (2010) The role of nucleotide sugar transporters in development of eukaryotes. Semin Cell Dev Biol 21(6):600–608
Lübke T, Marquardt T, Etzioni A, Hartmann E, von Figura K, Korner C (2001) Complementation cloning identifies CDG-IIc, a new type of congenital disorder of glycosylation, as a GDP-fucose transporter deficiency. Nat Genet 28(1):73–76
Lühn K, Wild MK, Eckhardt M, Gerardy-Schahn R, Vestweber D (2001) The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet 28(1):69–72
Lyon MF (1961) Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190:372–373
Mandato C, Brive L, Miura Y et al (2006) Cryptogenic liver disease in four children: a novel congenital disorder of glycosylation. Pediatr Res 59(2):293–298
Marquardt T, Denecke J (2003) Congenital disorders of glycosylation: Review of their molecular bases, clinical presentations and specific therapies. Eur J Pediatr 162:359–379
Marquardt T, Lühn K, Srikrishna G, Freeze HH, Harms E, Vestweber D (1999) Correction of leukocyte adhesion deficiency type II with oral fucose. Blood 94(12):3976–3985
Martinez I, Duncker I, Dupre T et al (2005) Genetic complementation reveals a novel human congenital disorder of glycosylation of type II, due to inactivation of the Golgi CMP-sialic acid transporter. Blood 105(7):2671–2676
Maszczak-Seneczko D, Olczak T, Wunderlich L, Olczak M (2011) Comparative analysis of involvement of UGT1 and UGT2 splice variants of UDP-galactose transporter in glycosylation of macromolecules in MDCK and CHO cell lines. Glycoconj J 28:481–492
Nakamura N, Rabouille C, Watson R et al (1995) Characterization of cis-Golgi matrix protein, GM130. J Cell Biol 131:1715–1726
Ng BG, Buckingham KJ, Raymond K et al (2013) Mosaicism of the UDP-galactose transporter SLC35A2 causes a congenital disorder of glycosylation. Am J Hum Genet 92:632–636
Niehues R, Hasilik M, Alton G et al (1998) Carbohydrate-deficient glycoprotein syndrome type Ib. Phosphomannose isomerase deficiency and mannose therapy. J Clin Invest 101:1414–1420
Olczak M, Guillen E (2006) Characterization of a mutation and an alternative splicing of UDP-galactose transporter in MDCK-RCAr cell line. Biochim Biophys Acta 1763(1):82–92
Olczak M, Maszczak-Seneczko D, Sosicka P, Jakimowicz P, Olczak T (2013) UDP Gal/UDP-GlcNAc chimeric transporter complements mutation defect in mammalian cells deficient in UDP-Gal transporter. Biochem Biophys Res Commun 434(3):473–478
Song Z (2013) Roles of the nucleotide sugar transporters (SLC35 family) in health and disease. Mol Asp Med 34:590–600
Sprong H, Degroote S, Nilsson T et al (2003) Association of the Golgi UDP-galactose transporter with UDP-galactose:ceramide galactosyltransferase allows UDP-galactose import in the endoplasmic reticulum. Mol Biol Cell 14(9):3482–3493
Stanley P (1989) Chinese hamster ovary cell mutants with multiple glcosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol Cell Biol 9(2):377–383
Tegtmeyer LC, Rust S, van Scherpenzeel M et al (2014) Multiple phenotypes in phosphoglucomutase 1 deficiency. N Engl J Med 370:533–542
Vallot C, Rougeulle C (2013) Inactivation du chromosome X chez l’humain XACT et XIST,à chacun son chromosome. Médecine/Sciences 29(2):223–225
Wada Y, Tajiri M, Yoshida S (2004) Hydrophilic affinity isolation and MALDI multiple-stage tandem mass spectrometry of glycopeptides for glycoproteomics. Anal Chem 76(22):6560–6565
Acknowledgments
We thank Maria Plate, Martina Herting and Ingrid Du Chesne for technical assistance. Vitaflo is acknowledged for providing D-galactose for oral supplementation.
Compliance with ethics guidelines
ᅟ
Conflict of interest
None.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000(5). Informed consent was obtained from all patients for being included in the study.
Additional informed consent was obtained from all patients for whom identifying information is included in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Eva Morava
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 48 kb)
ESM 2
(GIF 583 kb)
High Resolution Image
(TIFF 1277 kb)
ESM 3ESM 4
(GIF 984 kb)
(GIF 1301 kb)
High Resolution Image
(TIFF 493 kb)
High Resolution Image
(TIFF 248 kb)
ESM 5
(GIF 106 kb)
High Resolution Image
(TIFF 3538 kb)
ESM 6
(GIF 141 kb)
High Resolution Image
(TIFF 1912 kb)
ESM 7
(GIF 128 kb)
High Resolution Image
(TIFF 1144 kb)
Rights and permissions
About this article
Cite this article
Dörre, K., Olczak, M., Wada, Y. et al. A new case of UDP-galactose transporter deficiency (SLC35A2-CDG): molecular basis, clinical phenotype, and therapeutic approach. J Inherit Metab Dis 38, 931–940 (2015). https://doi.org/10.1007/s10545-015-9828-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-015-9828-6