Abstract
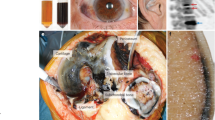
Alkaptonuria (AKU) is due to excessive homogentisic acid accumulation in body fluids due to lack of enzyme homogentisate dioxygenase leading in turn to varied clinical manifestations mainly by a process of conversion of HGA to a polymeric melanin-like pigment known as ochronosis. A potential treatment, a drug called nitisinone, to decrease formation of HGA is available. However, successful demonstration of its efficacy in modifying the natural history of AKU requires an effective quantitative assessment tool. We have described two potential tools that could be used to quantitate disease burden in AKU. One tool describes scoring the clinical features that includes clinical assessments, investigations and questionnaires in 15 patients with AKU. The second tool describes a scoring system that only includes items obtained from questionnaires used in 44 people with AKU. Statistical analyses were carried out on the two patient datasets to assess the AKU tools; these included the calculation of Chronbach’s alpha, multidimensional scaling and simple linear regression analysis. The conclusion was that there was good evidence that the tools could be adopted as AKU assessment tools, but perhaps with further refinement before being used in the practical setting of a clinical trial.









Similar content being viewed by others
References
Beck M (2006) The Mainz Severity Score Index (MSSI): development and validation of a system for scoring the signs and symptoms of Fabry disease. Acta Pædiatrica Suppl 451:43–46
Bland JM, Altman DG (1997) Statistical Notes: Chronbach’s alpha. BMJ 314:572
Committee for Medicinal Products for Human Use (CHMP) (2006) Guidelines on clinical trials in small populations (CHMP/EWP/83561/2005). European Medicines Agency, London
Cox TF, Cox MAA (2001) Multidimensional Scaling. Chapman & Hall, Boca Raton
D’Agostino RB (2009) The delayed-start study design. N Engl J Med 361(13):1304–1306
Fernandez-Canon JM, Granadino B, Beltran-Valero de Bernabe D et al. (1996) The molecular basis of alkaptonuria. Nat Genet 14:19–24
Gerss JWO, Köpcke W (2010) Clinical trials and rare diseases. In: Paz M, Groft SC (eds) Rare Diseases Epidemiology (Advances in Experimental Medicine and Biology 686). Springer, Dordrecht, pp 173–190
Goodfellow RJ, Schwartz J, Leya F (2005) Black aorta: a rare finding at aortic valve replacement. J Invasive Cardiol 17:165–167
Gower JC (1971) A general coefficient of similarity and some of its properties. Biometrics 27:857–874
Helliwell TR, Gallagher JA, Ranganath L (2008) Alkaptonuria - a review of surgical and autopsy pathology. Histopathol 53:503–512
Lagakos SW (2003) Clinical trials and rare diseases. N Engl J Med 348(24):2455–2456
O’Brien WM, La Du BN, Bunim JJ (1963) Biochemical, pathologic and clinical aspects of alcaptonuria, ochronosis and ochronotic arthropathy: review of world literature (1584–1962). Am J Med 34:813–838
Phornphutkul C, Introne WJ, Perry MB et al (2002) Natural history of alkaptonuria. N Engl J Med 347:2111–2121
Ranganath LR, Cox TF (2011) Natural history of alkaptonuria revisited: analyses based on scoring systems. JIMD (in press)
Wilke A, Steverding D (2009) Ochronosis as an unusual cause of valvular defect: a case report. J Med Case Reports 3:9302
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Ertan Mayatepek
Competing interest: None declared.
Rights and permissions
About this article
Cite this article
Cox, T.F., Ranganath, L. A quantitative assessment of alkaptonuria. J Inherit Metab Dis 34, 1153–1162 (2011). https://doi.org/10.1007/s10545-011-9367-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-011-9367-8