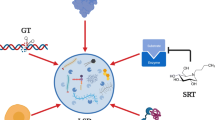
Abstract
Lysosomal storage disorders (LSD) are monogenic diseases caused by the deficiency of different lysosomal enzymes that degrade complex substrates such as glycosaminoglycans, sphingolipids, and others. As a consequence there is multisystemic storage of these substrates. Most treatments for these disorders are based in the fact that most of these enzymes are soluble and can be internalized by adjacent cells via mannose-6-phosphate receptor. In that sense, these disorders are good candidates to be treated by somatic gene therapy based on cell microencapsulation. Here, we review the existing data about this approach focused on the LSD treatments, the advantages and limitations faced by these studies.

Similar content being viewed by others
References
Barsoum SC, Milgram W, Mackay W et al. (2003) Delivery of recombinant gene product to canine brain with the use of microencapsulation. J Lab Clin Med 142(6):399–413
Barton NW, Brady RO, Dambrosia JM et al. (1991) Replacement therapy for inherited enzyme deficiency–macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med 324(21):1464–1470
Baruch L, Benny O, Gilert A, Ukobnik M, Ben Itzhak O, Machluf M (2009) Alginate-PLL cell encapsulation system Co-entrapping PLGA-microspheres for the continuous release of anti-inflammatory drugs. Biomed Microdevices 11:1103–1113
Beck M (2010) Therapy for Lysosomal Storage Disorders. IUBMB Life 62(1):33–40
Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C, Luca G, Basta G, Calafiore R (2006) Preparation and in vitro and in vivo characterization of composite microcapsules for cell encapsulation. Int J Pharm. 31;324(1):27–36.
Bloch J, Bachoud-Lévi AC, Déglon N et al. (2004) Neuroprotective gene therapy for Huntington's disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study. Hum Gene Ther 15(10):968–975
Boelens JJ, Prasad VK, Tolar J, Wynn RF, Peters C (2010) Current international perspectives on hematopoietic stem cell transplantation for inherited metabolic disorders. Pediatr Clin N Am 57(1):123–145
Bressel T, Paz AH, Baldo G, Lima EOC, Matte U, Saraiva-Pereira M (2008) An effective device for generating alginate microcapsules. Genet Mol Biol 31:1
Burton BK, Guffon N, Roberts J, van der Ploeg AT, Jones SA (2010) Home treatment with intravenous enzyme replacement therapy with idursulfase for mucopolysaccharidosis type II - data from the Hunter Outcome Survey. Mol Genet Metab 101(2–3):123–129
Chang TM (1964) Semipermeable microcapsules. Science 146:524–525
Chang PL (1999) Encapsulation for somatic gene therapy. Ann N Y Acad Sci 18:(875):146–158. Mater Res
Chang PL, Van Raamsdonk JM, Hortelano G, Barsoum SC, MacDonald NC, Stockley TL (1999) The in vivo delivery of heterologous proteins by microencapsulated recombinant cells. Trends Biotechnol 17(2):78–83
Clarke LA, Wraith JE, Beck M et al. (2009) Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics 123:229–240
Consiglio A, Martino S, Dolcetta D et al. (2007) Metabolic correction in oligodendrocytes derived from metachromatic leukodystrophy mouse model by using encapsulated recombinant myoblasts. J Neurol Sci 255(1–2):7–16
Cotugno G, Tessitore A, Capalbo A et al. (2010) Different serum enzyme levels are required to rescue the various systemic features of the mucopolysaccharidoses. Hum Gene Ther 21(5):555–569
Déglon N, Heyd B, Tan SA, Joseph JM, Zurn AD, Aebischer P (1996) Central nervous system delivery of recombinant ciliary neurotrophic factor by polymer encapsulated differentiated C2C12 myoblasts. Hum Gene Ther 7(17):2135–2146
Filoni C, Caciotti A, Carraresi L et al. (2010) Functional studies of new GLA gene mutations leading to conformational Fabry disease. Biochim Biophys Acta 1802(2):247–252
Friso A, Tomanin R, Alba S et al. (2005) Reduction of GAG storage in MPS II mouse model following implantation of encapsulated recombinant myoblasts. J Gene Med 7(11):1482–1491
Gan Q, Wang T (2007) Chitosan nanoparticle as protein delivery carrier-systematic examination of fabrication conditions for efficient loading and release. Colloids Surf B Biointerfaces 59(1):24–34
Grandoso L, Ponce S, Manuel I et al. (2007) Long-term survival of encapsulated GDNF secreting cells implanted within the striatum of parkinsonized rats. Int J Pharm 343(1–2):69–78
Griffon DJ, Sedighi MR, Sendemir-Urkmez A, Stewart AA, Jamison R (2005) Evaluation of vacuum and dynamic cell seeding of polyglycolic acid and chitosan scaffolds for cartilage engineering. Am J Vet Res 66(4):599–605
Harmatz P, Whitley CB, Waber L et al. (2004) Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). J Pediatr 144(5):574–580
Heese BA (2008) Current strategies in the management of lysosomal storage Diseases. Semin Pediatr Neurol 15:119–126
Hobbs JR (1981) Bone marrow transplantation for inborn errors. Lancet 2(8249):735–739
Hoffman D, Breakefield XO, Short MP, Aebischer P (1993) Transplantation of a polymer-encapsulated cell line genetically engineered to release NGF. Exp Neurol 122(1):100–106
Jardim LB, Aesse F, Vedolin LM et al. (2006) White matter lesions in Fabry disease before and after enzyme replacement therapy: a 2-year follow-up. Arq Neuropsiquiatr 64(3B):711–717
Jung SC, Park ES, Choi EN, Kim CH, Kim SJ, Jin DK (2010) Characterization of a novel mucopolysaccharidosis type II mouse model and recombinant AAV2/8 vector-mediated gene therapy. Mol Cells 30(1):13–18
Kakkis ED, Muenzer J, Tiller GE et al. (2001) Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med 344(3):182–188
Kishima H, Poyot T, Bloch J et al. (2004) Encapsulated GDNF-producing C2C12 cells for Parkinson's disease: a pre-clinical study in chronic MPTP-treated baboons. Neurobiol Dis 16(2):428–439
Kuijlen JM, de Haan BJ, Helfrich W, de Boer JF, Samplonius D, Mooij JJ, de Vos P (2006) The efficacy of alginate encapsulated CHO-K1 single chain-TRAIL producer cells in the treatment of brain tumors. J Neurooncol 78(1):31–39
Lagranha VL, Baldo G, de Carvalho TG et al. (2008) In vitro correction of ARSA deficiency in human skin fibroblasts from metachromatic leukodystrophy patients after treatment with microencapsulated recombinant cells. Metab Brain Dis 23(4):469–484
Lin HY, Huang CH, Yu HC et al. (2010) Enzyme assay and clinical assessment in subjects with a Chinese hotspot late-onset Fabry mutation (IVS4 + 919G– > A). J Inherit Metab Dis 33(5):619–624
Lowry RB, Renwick DH (1971) Relative frequency of the Hurler and Hunter syndromes. N Engl J Med 28;284(4):221–2.
Mabe-Santana P (2006) La enfermedad de Krabbe y la leucodistrofia metacromática. In: Sanjurjo P, Baldellou A (eds.) Diagnóstico y tratamiento de las enfermedades metabólicas hereditárias: Ergon, 639–650
Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL (2006) Sulfatide is essencial for the maintenance of CNS myelin and axon struture. Glia 53:372–381
Martin RA (2007) Mucopolysaccharidosis Type II. In: Pagon RA, Bird TC, Dolan CR, Stephens K (eds.) GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle
Martins AM, Dualibi AP, Norato D et al. (2009) Guidelines for the management of mucopolysaccharidosis type I. J Pediatr 155(4 Suppl):S32–S46
Matzner U, Herbst E, Hedayati KK et al. (2005) Enzyme replacement improves nervous system pathology and function in a mouse model for metachromatic leukodystrophy. Hum Mol Genet 14:1139–1152
Mayer FQ, Baldo G, de Carvalho TG, Lagranha VL, Giugliani R, Matte U (2010) Effects of cryopreservation and hypothermic storage on cell viability and enzyme activity in recombinant encapsulated cells overexpressing alpha-L-iduronidase. Artif Organs 34(5):434–439
Maysinger D, Berezovskaya O, Fedoroff S (1996) The hematopoietic cytokine colony stimulating factor 1 is also a growth factor in the CNS: (II). Microencapsulated CSF-1 and LM-10 cells as delivery systems. Exp Neurol 141(1):47–56
Mazumder MA, Burke NA, Shen F, Potter MA, Stöver HD (2009) Core-cross-linked alginate microcapsules for cell encapsulation. Biomacromolecules 8(10(6)):1365–1373
Muenzer J, Wraith JE, Beck M et al. (2006) A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome). Genet Med 8(8):465–473
Murua A, Orive G, Hernandez RM, Pedraz JL (2010) EPO delivery by genetically engineered C2C12 myoblasts immobilized in microcapsules. Adv Exp Med Biol 670:54–67
Naganawa Y, Ohsugi K, Kase R, Date I, Sakuraba H, Sakuragawa N (2002) In vitro study of encapsulation therapy for Fabry disease using genetically engineered CHO cell line. Cell Transplant 11(4):325–329
Nakama H, Ohsugi K, Otsuki T et al. (2006) Encapsulation cell therapy for mucopolysaccharidosis type VII using genetically engineered immortalized human amniotic epithelial cells. Tohoku J Exp Med 209(1):23–32
National MPS Society, viewed August 14 2010, http://www.mpssociety.org
Neufeld EF, Muenzer J (2001) The mucopolysaccharidoses. In: Scriver C, Beaudet A, Sly W et al. (eds) The metabolic and molecular bases of inherited disease. McGraw Hill, New York, pp 3421–3452
Orive G, Ponce S, Hernandez RM, Gascon AR, Igartua M, Pedraz JL (2002) Biocompatibility of microcapsules for cell imobilization alaborated with different type of alginates. Biomaterials 23(18):3825–3831
Orive G, Gascon RA, Hernandez RM, Igartua M, Pedraz JL (2003) Cell microencapsulation technology for biomedical purposes: novel insights and challenges. Trends Pharmacol Sci 24(5):207–210
Orive G, Tam SK, Pedraz JL, Hallé JP (2006) Biocompatibility of alginate-poly-L-lysine microcapsules for cell therapy. Biomaterials 27(20):3691–3700
Parkinson-Lawrence EJ, Shandala T, Prodoehl M, Plew R, Borlace GN, Brooks DA (2010) Lysosomal storage disease: revealing lysosomal function and physiology. Physyology 25:102–115
Peirone M, Ross CJ, Hortelano G, Brash JL, Chang PL (1998) Encapsulation of various recombinant mammalian cell types in different alginate microcapsules. J Biomed Mater Res 15(42(4)):587–96
Richard M, Arfi A, Seguin J, Gandolphe C, Scherman D (2009) Widespread biochemical correction of murine mucopolysaccharidosis type VII pathology by liver hydrodynamic plasmid delivery. Gene Ther 16(6):746–756
Ross CJ, Bastedo L, Maier SA, Sands MS, Chang PL (2000a) Treatment of a lysosomal storage disease, mucopolysaccharidosis VII, with microencapsulated recombinant cells. Hum Gene Ther 11(15):2117–2127
Ross CJ, Ralph M, Chang PL (2000b) Somatic gene therapy for a neurodegenerative disease using microencapsulated recombinant cells. Exp Neurol 166(2):276–286
Schiffmann R, Kopp JB, Austin HA et al. (2001) Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA 285(21):2743–2749
Shull RM, Kakkis ED, McEntee MF, Kania SA, Jonas AJ, Neufeld EF (1994) Enzyme replacement in a canine model of Hurler syndrome. Proc Natl Acad Sci USA 91(26):12937–12941
Shull RM, Lu X, McEntee MF, Bright RM, Pepper KA, Kohn DB (1996) Myoblast gene therapy in canine mucopolysaccharidosis. I: abrogation by an immune response to alpha-L-iduronidase. Hum Gene Ther 7(13):1595–1603
Sifuentes M, Doroshow R, Hoft R et al. (2007) A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years. Mol Genet Metab 90:171–180
Sirrs S, Clarke JT, Bichet DG et al. (2010) Baseline characteristics of patients enrolled in the Canadian Fabry Disease Initiative. Mol Genet Metab 99(4):367–373
Sly WS, Fischer HD, Gonzalez-Noriega A, Grubb JH, Natowicz M (1981) Role of the 6-phosphomannosyl-enzyme receptor in intracellular transport and adsorptive pinocytosis of lysosomal enzymes. Meth Cell Biol 23:191–214
Spuch C, Antequera D, Portero A et al. (2010) The effect of encapsulated VEGF-secreting cells on brain amyloid load and behavioral impairment in a mouse model of Alzheimer's disease. Biomaterials 31(21):5608–5618
Stein C, Gieselmann V, Kreysing J et al. (1989) Cloning and expression of human arylsulfatase A. J Biol Chem 264:1252–1259
Tomanin R, Friso A, Alba S et al. (2002) M. Non-viral transfer approaches for the gene therapy of mucopolysaccharidosis type II (Hunter syndrome). Acta Paediatr Suppl 91(439):100–104
Tylki-Szymanska A, Marucha J, Jurecka A, Syczewska M, Czartoryska M (2010) Efficacy of recombinant human α-L-iduronidase (laronidase) on restricted range of motion of upper extremities in mucopolysaccharidosis type I patients. J Inherit Metab Dis 33:151–157
Uludag H, Vos P, Tresco P (2000) Technology of mammalian cell encapsulation. Advac Drug Deliv Rev 42:29–64
Van den Hout JM, Reuser AJ, de Klerk JB, Arts WF, Smeitink JA, Van der Ploeg AT (2001) Enzyme therapy for pompe disease with recombinant human alpha-glucosidase from rabbit milk. J Inherit Metab Dis 24(2):266–274
Vitner EB, Platt FM, Futerman AH (2010) Common and uncommon pathogenic cascades in lysosomal storage diseases. J Biol Chem 285(27):20423–20427
Walkley SU (2009) Pathogenic cascades in lysosomal disease-Why so complex? J Inherit Metab Dis 32(2):181–189
Wraith JE (1995) The mucopolysaccharidoses: a clinical review and guide to management. Arch Dis Child 72:263–267
Acknowledgements
TGC is the recipient of a CAPES scholarship; VLL and FQM are recipients of CNPq scholarships, UM and RG are recipient of CNPq PQ scholarships. This work was supported by FIPE-HCPA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Gregory M. Pastores
Competing interest: None declared.
Rights and permissions
About this article
Cite this article
Matte, U., Lagranha, V.L., de Carvalho, T.G. et al. Cell microencapsulation: a potential tool for the treatment of neuronopathic lysosomal storage diseases. J Inherit Metab Dis 34, 983–990 (2011). https://doi.org/10.1007/s10545-011-9350-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-011-9350-4