Summary
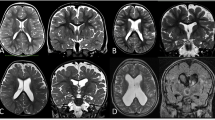
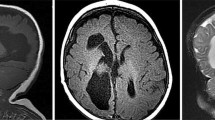
Patients with defects in the biogenesis of peroxisomes include those with Zellweger syndrome spectrum (ZSS), a developmental and progressive metabolic disease with a distinct dysmorphic phenotype and varying severity. The diagnosis of ZSS relies on the clinical presentation and the biochemical evaluation of peroxisomal metabolites. Mutation detection in one out of twelve genes coding for proteins involved in the biogenesis of peroxisomes confirms the diagnosis. In the absence of pronounced clinical features of ZSS, neuroradiological findings may lead the way to the diagnosis. Cerebral magnetic resonance imaging (cMRI) pathology in ZSS consists of abnormal gyration pattern including polymicrogyria and pachygyria, leukencephalopathy, germinolytic cysts and heterotopias as reported by previous systematic studies including cMRI of a total of 34 ZSS patients, only five of whom had a severe phenotype. The present study evaluated the cMRI results of additional 18 patients, 6 with a severe and 12 with a milder ZSS phenotype. It confirms and extends knowledge of the characteristic cMRI pattern in ZSS patients. Besides an abnormal gyration pattern and delayed myelination or leukencephalopathy, brain atrophy was a common finding. Polymicrogyria and pachygyria were more common in patients with severe ZSS, while leukencephalopathy increases with age in patients with longer survival. Nevertheless, an abnormal gyration pattern might be more frequent in patients with a mild ZSS than deduced from previous studies. In addition, we discuss the differential diagnosis of the ZSS cMRI pattern and review investigations on the pathogenesis of the ZSS cerebral phenotype in mouse models of the disease.



Similar content being viewed by others
Abbreviations
- ALD:
-
X-linked adrenoleukodystrophy
- cMRI:
-
cerebral magnetic resonance imaging
- IRD:
-
infantile Refsum disease
- MRS:
-
magnetic resonance spectroscopy
- NALD:
-
neonatal adrenoleukodystrophy
- PBD:
-
peroxisome biogenesis disorder
- RCDP:
-
rhizomelic chondrodysplasia punctata
- VLCFA:
-
very long-chain fatty acid
- ZS:
-
Zellweger syndrome
- ZSS:
-
Zellweger syndrome spectrum
References
Baes M, Gressens P, Baumgart E et al (1997) A mouse model for Zellweger syndrome. Nat Genet 17: 49–57.
Baes M, Dewerchin M, Janssen A, Collen D, Carmeliet P (2002a) Generation of Pex5-loxP mice allowing the conditional elimination of peroxisomes. Genesis 32: 177–178.
Baes M, Gressens P, Huyghe S et al (2002b) The neuronal migration defect in mice with Zellweger syndrome (Pex5 knockout) is not caused by the inactivity of peroxisomal beta-oxidation. J Neuropathol Exp Neurol 61: 368–374.
Barkovich AJ, Peck WW (1997) MR of Zellweger syndrome. AJNR Am J Neuroradiol 18: 1163–1170.
Barth PG, Gootjes J, Bode H, Vreken P, Majoie CB, Wanders RJ (2001) Late onset white matter disease in peroxisome biogenesis disorder. Neurology 57: 1949–1955.
Barth PG, Majoie CB, Gootjes J et al (2004) Neuroimaging of peroxisome biogenesis disorders (Zellweger spectrum) with prolonged survival. Neurology 62: 439–444.
Baumgart E, Vanhorebeek I, Grabenbauer M et al (2001) Mitochondrial alterations caused by defective peroxisomal biogenesis in a mouse model for Zellweger syndrome (PEX5 knockout mouse). Am J Pathol 159: 1477–1494.
Bjorkman J, Tonks I, Maxwell MA, Paterson C, Kay GF, Crane DI (2002) Conditional inactivation of the peroxisome biogenesis Pex13 gene by Cre-loxP excision. Genesis 32: 179–180.
Bruhn H, Kruse B, Korenke GC et al (1992) Proton NMR spectroscopy of cerebral metabolic alterations in infantile peroxisomal disorders. J Comput Assist Tomogr 16: 335–344.
de Vries LS, Gunardi H, Barth PG, Bok LA, Verboon-Maciolek MA, Groenendaal F (2004) The spectrum of cranial ultrasound and magnetic resonance imaging abnormalities in congenital cytomegalovirus infection. Neuropediatrics 35: 113–119.
Dirkx R, Vanhorebeek I, Martens K et al (2005) Absence of peroxisomes in mouse hepatocytes causes mitochondrial and ER abnormalities. Hepatology 41: 868–878.
Faust PL (2003) Abnormal cerebellar histogenesis in PEX2 Zellweger mice reflects multiple neuronal defects induced by peroxisome deficiency. J Comp Neurol 461: 394–413.
Faust PL, Hatten ME (1997) Targeted deletion of the PEX2 peroxisome assembly gene in mice provides a model for Zellweger syndrome, a human neuronal migration disorder. J Cell Biol 139: 1293–1305.
Faust PL, Su HM, Moser A, Moser HW (2001) The peroxisome deficient PEX2 Zellweger mouse: pathological and biochemical correlates of lipid dysfunction. J Mol Neurosci 16: 289–297; discussion 317–221.
Faust PL, Banka D, Siriratsivawong R, Ng VG, Wikander TM (2005) Peroxisome biogenesis disorders: the role of peroxisomes and metabolic dysfunction in developing brain. J Inherit Metab Dis 28: 369–383.
Ferdinandusse S, Denis S, Mooyer PA et al (2006) Clinical and biochemical spectrum of D-bifunctional protein deficiency. Ann Neurol 59: 92–104.
Garavelli L, Guareschi E, Errico S et al (2007) Megalencephaly and perisylvian polymicrogyria with postaxial polydactyly and hydrocephalus (MPPH): report of a new case. Neuropediatrics 38: 200–203.
Godfrey C, Clement E, Mein R et al (2007) Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain 130: 2725–2735.
Goh S (2007) Neuroimaging features in a neonate with rhizomelic chondrodysplasia punctata. Pediatr Neurol 37: 382–384.
Gressens P, Baes M, Leroux P et al (2000) Neuronal migration disorder in Zellweger mice is secondary to glutamate receptor dysfunction. Ann Neurol 48: 336–343.
Groenendaal F, Bianchi MC, Battini R et al (2001) Proton magnetic resonance spectroscopy (1H-MRS) of the cerebrum in two young infants with Zellweger syndrome. Neuropediatrics 32: 23–27.
Guerrini R (2005) Genetic malformations of the cerebral cortex and epilepsy. Epilepsia 46(Supplement 1): 32–37.
Janssen A, Baes M, Gressens P, Mannaerts GP, Declercq P, Van Veldhoven PP (2000) Docosahexaenoic acid deficit is not a major pathogenic factor in peroxisome-deficient mice. Lab Invest 80: 31–35.
Janssen A, Gressens P, Grabenbauer M et al (2003) Neuronal migration depends on intact peroxisomal function in brain and in extraneuronal tissues. J Neurosci 23: 9732–9741.
Kassmann CM, Lappe-Siefke C, Baes M et al (2007) Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat Genet 39: 969–976.
Kingsley PB, Shah TC, Woldenberg R (2006) Identification of diffuse and focal brain lesions by clinical magnetic resonance spectroscopy. NMR Biomed 19: 435–462.
Krysko O, Hulshagen L, Janssen A et al (2007) Neocortical and cerebellar developmental abnormalities in conditions of selective elimination of peroxisomes from brain or from liver. J Neurosci Res 85: 58–72.
Li X, Baumgart E, Dong GX et al (2002a) PEX11alpha is required for peroxisome proliferation in response to 4-phenylbutyrate but is dispensable for peroxisome proliferator-activated receptor alpha-mediated peroxisome proliferation. Mol Cell Biol 22: 8226–8240.
Li X, Baumgart E, Morrell JC, Jimenez-Sanchez G, Valle D, Gould SJ (2002b) PEX11 beta deficiency is lethal and impairs neuronal migration but does not abrogate peroxisome function. Mol Cell Biol 22: 4358–4365.
Malm G, Engman ML (2007) Congenital cytomegalovirus infections. Semin Fetal Neonatal Med 12: 154–159.
Martin PT (2006) Mechanisms of disease: congenital muscular dystrophies-glycosylation takes center stage. Nat Clin Pract Neurol 2: 222–230.
Maxwell M, Bjorkman J, Nguyen T et al (2003) Pex13 inactivation in the mouse disrupts peroxisome biogenesis and leads to a Zellweger syndrome phenotype. Mol Cell Biol 23: 5947–5957.
Mochel F, Grebille AG, Benachi A et al (2006) Contribution of fetal MR imaging in the prenatal diagnosis of Zellweger syndrome. AJNR Am J Neuroradiol 27: 333–336.
Moser HW, Mahmood A, Raymond GV (2007) X-linked adrenoleukodystrophy. Nat Clin Pract Neurol 3: 140–151.
Nakai A, Shigematsu Y, Nishida K, Kikawa Y, Konishi Y (1995) MRI findings of Zellweger syndrome. Pediatr Neurol 13: 346–348.
Ribeiro Mdo C, Gama de Sousa S, Freitas MM, Carrilho I, Fernandes I (2007) Bilateral perisylvian polymicrogyria and chromosome 1 anomaly. Pediatr Neurol 36: 418–420.
Rosewich H, Waterham HR, Wanders RJ et al (2006) Pitfall in metabolic screening in a patient with fatal peroxisomal beta-oxidation defect. Neuropediatrics 37: 95–98.
Staudt M, Krageloh-Mann I, Grodd W (2000) Normal myelination in childhood brains using MRI—a meta analysis. Rofo 172: 802–811.
Stone JA, Castillo M (1998) MR in a patient with Zellweger syndrome presenting without cortical or myelination abnormalities. AJNR Am J Neuroradiol 19: 1378–1379.
ter Rahe BS, Majoie CB, Akkerman EM, den Heeten GJ, Poll-The BT, Barth PG (2004) Peroxisomal biogenesis disorder: comparison of conventional MR imaging with diffusion-weighted and diffusion-tensor imaging findings. AJNR Am J Neuroradiol 25: 1022–1027.
van der Knaap MS, Valk J (1991) The MR spectrum of peroxisomal disorders. Neuroradiology 33: 30–37.
van der Knaap MS, Vermeulen G, Barkhof F, Hart AA, Loeber JG, Weel JF (2004) Pattern of white matter abnormalities at MR imaging: use of polymerase chain reaction testing of Guthrie cards to link pattern with congenital cytomegalovirus infection. Radiology 230: 529–536.
Vanhorebeek I, Baes M, Declercq PE (2001) Isoprenoid biosynthesis is not compromised in a Zellweger syndrome mouse model. Biochim Biophys Acta 1532: 28–36.
Viola A, Confort-Gouny S, Ranjeva JP et al (2002) MR imaging and MR spectroscopy in rhizomelic chondrodysplasia punctata. AJNR Am J Neuroradiol 23: 480–483.
Wanders RJ, Waterham HR (2006) Peroxisomal disorders: the single peroxisomal enzyme deficiencies. Biochim Biophys Acta 1763: 1707–1720.
Weller S, Gould SJ, Valle D (2003) Peroxisome biogenesis disorders. Annu Rev Genomics Hum Genet 4: 165–211.
Williams DW 3rd, Elster AD, Cox TD (1991) Cranial MR imaging in rhizomelic chondrodysplasia punctata. AJNR Am J Neuroradiol 12: 363–365.
Young S, Rabi Y, Lodha AK (2007) Band heterotopia in Zellweger syndrome (cerebro-hepato-renal syndrome). Neurol India 55: 93.
Acknowledgements
We thank Professor Volkher Engelbrecht, Department of Radiology, Klinikum St. Marien Amberg, Germany, for help in evaluating part of the cMRIs. This work was supported by the Deutsche Forschungsgemeinschaft, grants nos. GA354/5–1 and 5–2 (J.G.), the Fritz Thyssen-Stiftung grant no. Az.10.05.2.197 (S.W.) and the Faculty of Medicine research grant from the Georg August University Göttingen (S.W., H.R.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicating editor: Verena Peters
Competing interests: None declared
References to electronic databases: Peroxisome biogenesis disorder: OMIM #601539. Zellweger syndrome: OMIM #214100. Neonatal adrenoleukodystrophy: OMIM #202370. Infantile Refsum disease: OMIM #266510
Rights and permissions
About this article
Cite this article
Weller, S., Rosewich, H. & Gärtner, J. Cerebral MRI as a valuable diagnostic tool in Zellweger spectrum patients. J Inherit Metab Dis 31, 270–280 (2008). https://doi.org/10.1007/s10545-008-0856-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-008-0856-3