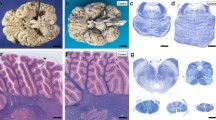
Summary
The neuronal ceroid lipofuscinoses (NCLs) are inherited neurodegenerative diseases characterized by massive accumulation of autofluorescent storage bodies in neurons and other cells. A late-onset form of NCL occurs in Tibetan terrier dogs. Gel electrophoretic analyses of isolated storage body proteins from brains of affected dogs indicated that a protein of approximately 50 kDa was consistently prominent and a 16 kDa component was present in some brain storage body preparations. Mass spectral analysis identified the 50 kDa protein as glial fibrillary acidic protein (GFAP), isoform 2. GFAP identification was supported by immunoblot and immunohistochemical analyses. Histone H4 was the major protein in the 16 kDa component. Specific accumulation of GFAP and histone H4 in storage bodies has not been previously reported for any of the NCLs. Tibetan terrier NCL may be the canine correlate of one of the human adult-onset NCLs for which the genetic bases and storage body compositions have not yet been determined.
Similar content being viewed by others
References
Asahina N, Okamoto T, Sudo A, Kanazawa N, Tsujino S, Saitoh S (2006) An infantile-juvenile form of Alexander disease caused by a R79H mutation in GFAP. Brain Dev 28: 131-33.
Awano T, Katz ML, O’Brien DP, et al (2006a) A mutation in the cathepsin D gene (CTSD) in American Bulldogs with neuronal ceroid-lipofuscinosis. Mol Genet Metab 87: 341-48.
Awano T, Katz ML, Sohar I, et al (2006b) A frame shift mutation in the canine ortholog of human CLN2 in a juvenile dachshund with neuronal ceroid lipofuscinosis. Mol Genet Metab 89: 254-60.
Buzy A, Ryan EM, Jennings KR, Palmer DN, Griffiths DE (1996) Use of electrospray ionization mass spectrometry to study binding of F0 inhibitors to ceroid lipofuscinosis protein, a model system for subunit c of mitochondrial ATP synthase. Rapid Commun Mass Spectrom 10: 790-96.
Condorelli DF, Nicoletti VG, Barresi V, et al (1999) Structural features of the rat GFAP gene and identification of a novel alternative transcript. J Neurosci Res 56: 219-28.
Drögemüller C, Wöhlke A, Distl O (2005) Evaluation of the canine TPP1 gene as a candidate for neuronal ceroid lipofuscinosis in Tibetan Terrier and Polish Owczarek Nizinny dogs. Animal Genet 36: 178-79.
Fearnley IM, Walker JE, Martinus RD, et al (1990) The sequence of the major protein stored in ovine ceroid-lipofuscinosis is identical with that of the dicyclohexylcarbodiimide-reactive proteolipid of mitochondrial ATP synthase. Biochem J 268: 751-58.
Fuchs E, Weber K (1994) Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem 63: 345-82.
Goebel HH (1996) The neuronal ceroid-lipofuscinoses. Semin Pediatr Neurol 3: 270-78.
Goebel HH (2000) Morphological aspects of the neuronal ceroid lipofuscinoses. Neurol Sci 21: S27–S33.
Goebel HH, Wisniewski KE (2004) Current state of clinical and morphological features in human NCL. Brain Pathol 14: 61-9.
Goebel HH, Zeman W, Patel VK, Pullarkat RK, Lenard HG (1979) On the ultrastructural diversity and essence of residual bodies in neuronal ceroid-lipofuscinosis. Mech Age Dev 10: 53-0.
Goebel HH, Braak H, Seidel D, Doshi R, Marsden CD, Gullotta F (1982) Morphologic studies on adult neuronal-ceroid lipofuscinosis (NCL). Clin Neuropathol 1: 151-62.
Hall NA, Lake BD, Dewji NN, Patrick AD (1991) Lysosomal storage of subunit c of mitochondrial ATP synthase in Batten’s disease (ceroid-lipofuscinosis). Biochem J 275: 269-72.
Haltia M (2003) The neuronal ceroid-lipofuscinoses. J Neuropathol Exp Neurol 62: 1-3.
Haltia M (2006) The neuronal ceroid-lipofuscinoses: from past to present. Biochim Biophys Acta 1762: 850-56.
Herva R, Tyynela J, Hirvasniemi A, Syrjakallio-Ylitalo M, Haltia M (2000) Northern epilepsy: a novel form of neuronal ceroid-lipofuscinosis. Brain Pathol 10: 215-22.
Hol EM, Roelofs RF, Moraal E, et al (2003) Neuronal expression of GFAP in patients with Alzheimer pathology and identification of novel GFAP splice forms. Mol Psychiatry 8: 786-96.
Hsiao VC, Tian R, Long H, et al (2005) Alexander-disease mutation of GFAP causes filament disorganization and decreased solubility of GFAP. J Cell Sci 118: 2057-065.
Ishigami A, Ohsawa T, Hiratsuka M, et al (2005) Abnormal accumulation of citrullinated proteins catalyzed by peptidylarginine deiminase in hippocampal extracts from patients with Alzheimer’s disease. J Neurosci Res 80: 120-28.
Johnson AB (2004) Alexander disease: a leukodystrophy caused by a mutation in GFAP. Neurochem Res 29: 961-64.
Jolly RD (1995) Comparative biology of the neuronal ceroid-lipofuscinoses (NCL): an overview. Am J Med Genet 57: 307-11.
Jolly RD, Palmer DN (1995) The neuronal ceroid-lipofuscinoses (Batten disease): comparative aspects. Neuropath Appl Neurobiol 21: 50-0.
Jolly RD, Martinus RD, Palmer DN (1992) Sheep and other animals with ceroid-lipofuscinosis: their relevance to Batten disease. Am J Med Genet 42: 609-14.
Jolly RD, Palmer DN, Studdert VP, et al (1994) Canine ceroid-lipofuscinosis: a review and classification. J Small Anim Pract 35: 299-06.
Katz ML, Christianson JS, Norbury NE, Gao C, Siakotos AN, Koppang N (1994) Lysine methylation of mitochondrial ATP synthase subunit c stored in tissues of dogs with hereditary ceroid-lipofuscinosis. J Biol Chem 269: 9906-911.
Katz ML, Gao C, Tompkins JA, Chin DT, Bronson RT (1995) Mitochondrial ATP synthase subunit c stored in hereditary ceroid-lipofuscinosis contains trimethyllysine. Biochem J 310: 887-92.
Katz ML, Siakotos AN, Gao Q, Freiha B, Chin DT (1997) Late-infantile ceroid-lipofuscinosis: lysine methylation of mitochondrial subunit c from lysosomal storage bodies. Biochim Biophys Acta 1361: 66-4.
Katz ML, Shibuya H, Johnson GS (2001) Animal models for the ceroid lipofuscinoses. Adv Genet 45: 183-03.
Katz ML, Sanders DA, Sanders DN, Hansen E, Johnson GS (2002) Assessment of plasma carnitine levels in Tibetan Terriers in relation to ceroid-lipofuscinosis. Am J Vet Res 63: 890-95.
Katz ML, Khan S, Awano T, Shahid A, Siakotos AN, Johnson GS (2005a) A mutation in the CLN8 gene in English Setter dogs with neuronal ceroid-lipofuscinosis. Biochem Biophys Res Commun 327: 541-47.
Katz ML, Narfström K, Johnson GS, O’Brien DP (2005b) Assessment of retinal function and characterization of lysosomal storage body accumulation in the retinas and brains of Tibetan Terriers with ceroid-lipofuscinosis. Am J Vet Res 66: 67-6.
Kominami E, Ezaki J, Muno D, Ishido K, Ueno T, Wolfe LS (1992) Specific storage of subunit c of mitochondrial ATP synthase in lysosomes of neuronal ceroid lipofuscinosis (Batten’s disease). J Biochem 111: 278-82.
Koppang N (1973) Canine ceroid-lipofuscinosis—a model for human neuronal ceroid-lipofuscinosis and aging. Mech Age Dev 2: 421-45.
Korolainen MA, Auriola S, Nyman TA, Alafuzoff I, Pirttila T (2005) Problems associated with biological markers of Alzheimer’s disease. Neurochem Res 30: 1501-510.
Li R, Johnson AB, Salomons G, et al (2005) Glial fibrillary acidic protein mutations in infantile, juvenile, and adult forms of Alexander disease. Ann Neurol 57: 310-26.
Martin JJ (1991) Adult type of neuronal ceroid lipofuscinosis. Dev Neurosci 13: 331-38.
Muntane G, Dalfo E, Martinez A, et al (2006) Glial fibrillary acidic protein is a major target of glycoxidative and lipoxidative damage in Pick’s disease. J Neurochem 99: 177-85.
Narfström K, Katz ML, Bragadottir R, et al (2003) Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci 44: 1663-672.
Neville H, Armstrong D, Wilson B, Koppang N, Wehling C (1980) Studies on the retina and the pigment epithelium in hereditary canine ceroid lipofuscinosis. Invest Ophthalmol Vis Sci 19: 75-6.
Nielsen AL, Holm IE, Johansen M, Bonven B, Jorgensen P, Jorgensen AL (2002) A new splice variant of glial fibrillary acidic protein, GFAP epsilon, interacts with the presenilin proteins. J Biol Chem 277: 29983-9991.
Nijssen PC, Ceuterick C, van Diggelen OP, et al (2003) Autosomal dominant adult neuronal ceroid lipofuscinosis: a novel form of NCL with granular osmiophilic deposits without palmitoyl protein thioesterase 1 deficiency. Brain Pathol 13: 574-81.
Palmer DN, Barns G, Husbands DR, Jolly RD (1986) Ceroid lipofuscinosis in sheep. II. The major component of the lipopigment in liver, kidney, pancreas, and brain is low molecular weight protein. J Biol Chem 261: 1773-777.
Palmer DN, Martinus RD, Cooper SM, Midwinter GG, Reid JC, Jolly RD (1989) Ovine ceroid-lipofuscinosis. The major lipopigment protein and the lipid-binding subunits of mitochondrial ATP synthase have the same NH2-terminal sequence. J Biol Chem 264: 5736-740.
Palmer DN, Fearnley IM, Medd SM, et al (1990) Lysosomal storage of the DCCD-reactive proteolipid subunit of mitochondrial ATP synthase in human and ovine ceroid-lipofuscinosis. In: Porta EA, ed. Lipofuscin and Ceroid Pigments. New York: Plenum, 211–223.
Palmer DN, Jolly RD, van Mil HC, Tyynela J, Westlake VJ (1997) Different patterns of hydrophobic protein storage in different forms of neuronal ceroid lipofuscinosis (NCL, Batten disease). Neuropediatrics 28: 45-8.
Ranta S, Savukoski M, Santavuori P, Haltia M (2001) Studies of homogeneous populations: CLN5 and CLN8. Adv Genet 45: 123-40.
Reeves SA, Helman LJ, Allison A, Israel MA (1989) Molecular cloning and primary structure of human glial fibrillary acidic protein. Proc Nat Acad Sci USA 86: 5178-182.
Riis RC, Cummings JF, Loew ER, de Lahunta A (1992) Tibetan terrier model of canine ceroid lipofuscinosis. Am J Med Genet 42: 615-21.
Seehafer SS, Pearce DA (2006) You say lipofuscin, we say ceroid: defining autofluorescent storage material. Neurobiol Aging 27: 576-88.
Siakotos AN, Schnippel K, Lin RC, Van Kuijk FJ (1995) Biosynthesis and metabolism of 4-hydroxynonenal in canine ceroid-lipofuscinosis. Am J Med Genet 57: 290-93.
Siintola E, Partanen S, Stromme P, et al (2006) Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain 129: 1438-445.
Siintola E, Topcu M, Aula N, et al (2007) The novel neuronal ceroid lipofuscinosis gene MFSD8 encodes a putative lysosomal transporter. Am J Human Genet 81: 136-46.
Singh R, Nielsen AL, Johansen MG, Jorgensen AL (2003) Genetic polymorphism and sequence evolution of an alternatively spliced exon of the glial fibrillary acidic protein gene, GFAP. Genomics 82: 185-93.
Steinfeld R, Reinhardt K, Schreiber K, et al (2006) Cathepsin D deficiency is associated with a human neurodegenerative disorder. Am J Human Genet 78: 988-98.
Takemura M, Gomi H, Colucci-Guyon E, Itohara S (2002) Protective role of phosphorylation in turnover of glial fibrillary acidic protein in mice. J Neurosci 22: 6972-979.
Tian R, Gregor M, Wiche G, Goldman JE (2006) Plectin regulates the organization of glial fibrillary acidic protein in Alexander disease. Am J Pathol 168: 888-97.
Tyynela J, Palmer DN, Baumann M, Haltia M (1993) Storage of saposins A and B in infantile neuronal ceroid-lipofuscinoses. FEBS Lett 330: 8-2.
Tyynela J, Suopanki J, Baumann M, Haltia M (1997a) Sphingolipid activator proteins (SAPs) in neuronal ceroid-lipofuscinosis (NCL). Pediatrics 28: 49-2.
Tyynela J, Suopanki J, Santavuori P, Baumann M, Haltia M (1997b) Variant late infantile ceroid-lipofuscinosis: pathology and biochemistry. J Neuropathol Exp Neurol 56: 369-75.
Wisniewski KE, Kida E, Golabek AA, Kaczmarski W, Zhong N (2001) Neuronal ceroid lipofuscinoses: classification and diagnosis. Adv Genet 45: 1-4.
Wöhlke A, Distl O, Drögemüller C (2005) The canine CTSD gene as a candidate for late-onset neuronal ceroid lipofuscinosis. Animal Genet 36: 530-32.
Wöhlke A, Distl O, Drögemüller C (2006) Characterization of the canine CLCN3 gene and evaluation as candidate for late-onset NCL. BMC Genet 7: 13.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicating editor: Joe Clarke
Competing interests: None declared
Rights and permissions
About this article
Cite this article
Katz, M.L., Sanders, D.N., Mooney, B.P. et al. Accumulation of glial fibrillary acidic protein and histone H4 in brain storage bodies of Tibetan terriers with hereditary neuronal ceroid lipofuscinosis. J Inherit Metab Dis 30, 952–963 (2007). https://doi.org/10.1007/s10545-007-0683-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-007-0683-y