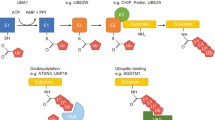
Summary
Newly synthesized proteins in the living cell must go through a folding process to attain their functional structure. To achieve this in an efficient fashion, all organisms, including humans, have evolved a large set of molecular chaperones that assist the folding as well as the maintenance of the functional structure of cellular proteins. Aberrant proteins, the result of production errors, inherited or acquired amino acid substitutions or damage, especially oxidative modifications, can in many cases not fold correctly and will be trapped in misfolded conformations. To rid the cell of misfolded proteins, the living cell contains a large number of intracellular proteases, e.g. the proteasome, which together with the chaperones comprise the cellular protein quality control systems. Many inherited disorders due to amino acid substitutions exhibit loss-of-function pathogenesis because the aberrant protein is eliminated by one of the protein quality control systems. Examples are cystic fibrosis and phenylketonuria. However, not all aberrant proteins can be eliminated and the misfolded protein may accumulate and form toxic oligomeric and/or aggregated inclusions. In this case the loss of function may be accompanied by a gain-of-function pathogenesis, which in many cases determines the pathological and clinical features. Examples are Parkinson and Huntington diseases. Although a number of strategies have been tried to decrease the amounts of accumulated and aggregated proteins, a likely future strategy seems to be the use of chemical or pharmacological chaperones with specific effects on the misfolded protein in question. Positive examples are enzyme enhancement in a number of lysosomal disorders.
Similar content being viewed by others
References
Anfinsen CB (1973) Principles that govern the folding of protein chains. Science 181: 223–230.
Bence NF, Sampat RM, Kopito RR (2001) Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292: 1552–1555.
Bernier V, Lagace M, Bichet DG, Bouvier M (2004) Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab 15: 222–228.
Beutler E, Grabowski GA (2001) Gaucher disease. In: Scriver CR, Beaudet al, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, eds. The Metabolic and Molecular Bases of Inherited Disease, 8th edn. New York: McGraw-Hill, 3635–3668.
Bruijn LI, Miller TM, Cleveland DW (2004) Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci 27: 723–749.
Brusilow SW, Horwich AL (2001) Urea cycle enzymes. In: Scriver CR, Beaudetal, Sly WS, Valle D, eds; Childs B, Kinzler KW, Vogelstein B, assoc, eds. The Metabolic and Molecular Bases of Inherited Disease, 8th edn. New York: McGraw-Hill, 1909–1963.
Bucciantini M, Calloni G, Chiti F, etal (2004) Prefibrillar amyloid protein aggregates share common features of cytotoxicity. J Biol Chem 279: 31374–31382.
Burch M, Blair E (1999) The inheritance of hypertrophic cardiomyopathy. Pediatr Cardiol 20: 313–316.
Burrows JA, Willis LK, Perlmutter DH (2000) Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: a potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc Natl Acad Sci USA 97: 1796–1801.
Butterfield DA, Kanski J (2001) Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev 122: 945–962.
Calloni G, Zoffoli S, Stefani M, Dobson CM, Chiti F (2005) Investigating the effects of mutations on protein aggregation in the cell. J Biol Chem 280: 10607–10613.
Carrell RW, Lomas DA (2002) Alpha1-antitrypsin deficiency—a model for conformational diseases. N Engl J Med 346: 45–53.
Casari G, De Fusco M, Ciarmatori S, etal (1998) Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial metalloprotease. Cell 93: 973–983.
Cashikar AG, Duennwald M, Lindquist SL (2005) A chaperone pathway in protein disaggregation. Hsp26 alters the nature of protein aggregates to facilitate reactivation by Hsp104. J Biol Chem 280: 23869–23875.
Caughey B, Lansbury PT (2003) Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26: 267–298.
Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM (2003) Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature 424: 805–808.
Christensen JH, Siggaard C, Corydon TJ, etal (2004) Impaired trafficking of mutated AVP prohormone in cells expressing rare disease genes causing autosomal dominant familial neurohypophyseal diabetes insipidus. Clin Endocrinol (Oxf) 60: 125–136.
Clarkson E, Costa CF, Machesky LM (2004) Congenital myopathies: diseases of the actin cytoskeleton. J Pathol 204: 407–417.
Cookson MR (2005) The biochemistry of Parkinson's disease. Annu Rev Biochem 74: 29–52.
Cornett J, Cao F, Wang CE, etal (2005) Polyglutamine expansion of huntingtin impairs its nuclear export. Nature Genetics 37: 198–204.
Corydon MJ, Vockley J, Rinaldo P, etal (2001) Role of common gene variations in the molecular pathogenesis of short-chain acyl-CoA dehydrogenase deficiency. Pediatr Res 49: 18–23.
Costa CF, Rommelaere H, Waterschoot D, etal (2004) Myopathy mutations in alpha-skeletal-muscle actin cause a range of molecular defects. J Cell Sci 117: 3367–3377.
Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A (2003) Protein carbonylation in human diseases. Trends Mol Med 9: 169–176.
Desnick RJ (2004) Enzyme replacement and enhancement therapies for lysosomal diseases. J Inherit Metab Dis 27: 385–410.
Dukan S, Farewell A, Ballesteros M, Taddei F, Radman M, Nystrom T (2000) Protein oxidation in response to increased transcriptional or translational errors. Proc Natl Acad Sci USA 97: 5746–5749.
Ellis RJ (1993) The general concept of molecular chaperones. Philos Trans R Soc Lond B Biol Sci 339: 257–261.
Ellis RJ, Pinheiro TJ (2002) Medicine: danger — misfolding proteins. Nature 416: 483–484.
Emerit J, Edeas M, Bricaire F (2004) Neurodegenerative diseases and oxidative stress. Biomed Pharmacother 58: 39–46.
Fan JQ (2003) A contradictory treatment for lysosomal storage disorders: inhibitors enhance mutant enzyme activity. Trends Pharmacol Sci 24: 355–360.
Farinha CM, Amaral MD (2005) Most F508del-CFTR is targeted to degradation at an early folding checkpoint and independently of calnexin. Mol Cell Biol 25: 5242–5252.
Foster BA, Coffey HA, Morin MJ, Rastinejad F (1999) Pharmacological rescue of mutant p53 conformation and function. Science 286: 2507–2510.
Friedlander RM (2003) Apoptosis and caspases in neurodegenerative diseases. N Engl J Med 348: 1365–1375.
Frydman J (2001) Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu Rev Biochem 70: 603–647.
Gamez A, Perez B, Ugarte M, Desviat LR (2000) Expression analysis of phenylketonuria mutations. Effect on folding and stability of the phenylalanine hydroxylase protein. J Biol Chem 275: 29737–29742.
Gelman MS, Kopito RR (2003) Cystic fibrosis: premature degradation of mutant proteins as a molecular disease mechanism. Methods Mol Biol 232: 27–37.
Gestwicki JE, Crabtree GR, Graef IA (2004) Harnessing chaperones to generate small-molecule inhibitors of amyloid beta aggregation. Science 306: 865–869.
Gregersen N, Winter VS, Corydon MJ, etal (1998) Identification of four new mutations in the short-chain acyl-CoA dehydrogenase (SCAD) gene in two patients: one of the variant alleles, 511CT, is present at an unexpectedly high frequency in the general population, as was the case for 625GA, together conferring susceptibility to ethylmalonic aciduria. Hum Mol Genet 7: 619–627.
Gregersen N, Andresen BS, Corydon MJ, etal (2001) Mutation analysis in mitochondrial fatty acid oxidation defects: exemplified by acyl-CoA dehydrogenase deficiencies, with special focus on genotype-phenotype relationship. Hum Mutat 18: 169–189.
Gregersen N, Bross P, Andresen BS (2004) Genetic defects in fatty acid beta-oxidation and acyl-CoA dehydrogenases. Eur J Biochem 271: 470–482.
Gregersen N, Bross P, Jórgensen MM (2005) Protein folding and misfolding: the role of cellular protein quality control systems in inherited disorders. In: Scriver CR, Beaudetal, Valle D, Sly WS, Childs B, Kinzler KW, Vogelstein B. MMBID-ONLINE: http://genetics.accessmedicine.com. McGraw-Hill, New York, Chapter 13.1.
Grune T, Jung T, Merker K, Davies KJ (2004) Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. Int J Biochem Cell Biol 36: 2519–2530.
Hansen JJ, Durr A, Cournu-Rebeix I, etal (2002) Hereditary spastic paraplegia SPG13 is associated with a mutation in the gene encoding the mitochondrial chaperonin Hsp60. Am J Hum Genet 70: 1328–1332.
Hayden MR, Kremer B (2001) Huntington disease. In: Scriver CR, Beaudetal, Sly WS, Valle D, eds; Childs B, Kinzler KW, Vogelstein B, assoc, eds. The Metabolic and Molecular Bases of Inherited Disease, 8th edn. New York: McGraw-Hill, 5677–5701.
Haynes CM, Titus EA, Cooper AA (2004) Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell 15: 767–776.
Ilkovski B, Nowak KJ, Domazetovska A, etal (2004) Evidence for a dominant-negative effect in ACTA1 nemaline myopathy caused by abnormal folding, aggregation and altered polymerization of mutant actin isoforms. Hum Mol Genet 13: 1727–1743.
Jakobsen LD, Jensen PH (2003) Parkinson's disease: alpha-synuclein and parkin in protein aggregation and the reversal of unfolded protein stress. Methods Mol Biol 232: 57–66.
Jana NR, Tanaka M, Wang G, Nukina N (2000) Polyglutamine length-dependent interaction of Hsp40 and Hsp70 family chaperones with truncated N-terminal huntingtin: their role in suppression of aggregation and cellular toxicity. Hum Mol Genet 9: 2009–2018.
Jorgensen MM, Bross P, Gregersen N (2003) Protein quality control in the endoplasmic reticulum. APMIS Suppl 86–91.
Kayed R, Head E, Thompson JL, etal (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300: 486–489.
Kopito RR (2000) Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol 10: 524–530.
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6: 463–477.
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22: 631–677.
Lotharius J, Brundin P (2002) Impaired dopamine storage resulting from alpha-synuclein mutations may contribute to the pathogenesis of Parkinson's disease. Hum Mol Genet 11: 2395–2407.
Maquat LE (2005) Nonsense-mediated mRNA decay in mammals. J Cell Sci 118: 1773–1776.
Milewski MI, Mickle JE, Forrest JK, Stanton BA, Cutting GR (2002) Aggregation of misfolded proteins can be a selective process dependent upon peptide composition. J Biol Chem 277: 34462–34470.
Miller TW, Messer A (2005) Intrabody applications in neurological disorders: progress and future prospects. Mol Ther 12: 394–401.
Miller VM, Xia H, Marrs GL, etal (2003) Allele-specific silencing of dominant disease genes. Proc Natl Acad Sci USA 100: 7195–7200.
Morello JP, Salahpour A, Petaja-Repo UE, etal (2001) Association of calnexin with wild type and mutant AVPR2 that causes nephrogenic diabetes insipidus. Biochem 40: 6766–6775.
Morishima N (2005) Control of cell fate by Hsp70: more than an evanescent meeting. J Biochem (Tokyo) 137: 449–453.
Muchowski PJ, Walker JL (2005) Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci 6: 11–22.
Nagan N, Kruckeberg KE, Tauscher AL, Snow BK, Rinaldo P, Matern D (2003) The frequency of short-chain acyl-CoA dehydrogenase gene variants in the US population and correlation with the C(4)-acylcarnitine concentration in newborn blood spots. Mol Genet Metab 78: 239–246.
Okado-Matsumoto A, Fridovich I (2002) Amyotrophic lateral sclerosis: a proposed mechanism. Proc Natl Acad Sci USA 99: 9010–9014.
Pedersen CB, Bross P, Winter VS, etal (2003) Misfolding, degradation, and aggregation of variant proteins. The molecular pathogenesis of short chain acyl-CoA dehydrogenase (SCAD) deficiency. J Biol Chem 278: 47449–47458.
Perlmutter DH (2002) Chemical chaperones: a pharmacological strategy for disorders of protein folding and trafficking. Pediatr Res 52: 832–836.
Perlmutter DH (2003) Alpha1-antitrypsin deficiency: liver disease associated with retention of a mutant secretory glycoprotein in the endoplasmic reticulum. Methods Mol Biol 232: 39–56.
Pey AL, Desviat LR, Gamez A, Ugarte M, Perez B (2003) Phenylketonuria: genotype—phenotype correlations based on expression analysis of structural and functional mutations in PAH. Hum Mutat 21: 370–378.
Qin ZH, Gu ZL (2004) Huntingtin processing in pathogenesis of Huntington disease. Acta Pharmacol Sin 25: 1243–1249.
Rattan SI (2004) Hormetic mechanisms of anti-aging and rejuvenating effects of repeated mild heat stress on human fibroblasts in vitro. Rejuvenation Res 7: 40–48.
Ron I, Horowitz M (2005) ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet 14: 2387–2398.
Rubenstein RC, Zeitlin PL (2000) Sodium 4-phenylbutyrate downregulates Hsc70: implications for intracellular trafficking of DeltaF508-CFTR. Am J Physiol Cell Physiol 278: C259—C267.
Rubenstein RC, Egan ME, Zeitlin PL (1997) In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J Clin Invest 100: 2457–2465.
Saarela J, Laine M, Oinonen C, etal (2001) Molecular pathogenesis of a disease: structural consequences of aspartylglucosaminuria mutations. Hum Mol Genet 10: 983–995.
Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW (2002) Chemical chaperones increase the cellular activity of N370S beta-glucosidase: a therapeutic strategy for Gaucher disease. Proc Natl Acad Sci USA 99: 15428–15433.
Schon EA, Manfredi G (2003) Neuronal degeneration and mitochondrial dysfunction. J Clin Invest 111: 303–312.
Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR (2000) Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404: 770–774.
Scriver CR, Kaufman S (2001) Hyperphenylalanemia: phenylalenine hydroxylase deficiency. In: Scriver CR, Beaudetal, Sly WS, Valle D, eds; Childs B, Kinzler KW, Vogelstein B, assoc, eds. The Metabolic and Molecular Bases of Inherited Disease, 8th edn. New York: McGraw-Hill, 1667–1724.
Sherman MY, Goldberg AL (2001) Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron 29: 15–32.
Smith MA, Drew KL, Nunomura A, etal (2002) Amyloid-beta, tau alterations and mitochondrial dysfunction in Alzheimer disease: the chickens or the eggs? Neurochem Int 40: 527–531.
Sorensen CB, Andresen BS, Jensen UB, etal (2003) Functional testing of keratin 14 mutant proteins associated with the three major subtypes of epidermolysis bullosa simplex. Exp Dermatol 12: 472–479.
Stefani M, Dobson CM (2003) Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med 81: 678–699.
Stone DL, Slavotinek A, Bouffard GG, etal (2000) Mutation of a gene encoding a putative chaperonin causes McKusick—Kaufman syndrome. Nature Genetics 25: 79–82.
Suhr ST, Senut MC, Whitelegge JP, Faull KF, Cuizon DB, Gage FH (2001) Identities of sequestered proteins in aggregates from cells with induced polyglutamine expression. J Cell Biol 153: 283–294.
Tanaka M, Machida Y, Nukina N (2005) A novel therapeutic strategy for polyglutamine diseases by stabilizing aggregation-prone proteins with small molecules. J Mol Med 83: 343–352.
Teckman JH (2004) Lack of effect of oral 4-phenylbutyrate on serum alpha-1-antitrypsin in patients with alpha-1-antitrypsin deficiency: a preliminary study. J Pediatr Gastroenterol Nutr 39: 34–37.
Trinklein ND, Murray JI, Hartman SJ, Botstein D, Myers RM (2004) The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol Biol Cell 15: 1254–1261.
Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM (2004) Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic 5: 821–837.
Uversky VN (2002) Natively unfolded proteins: a point where biology waits for physics. Protein Sci 11: 739–756.
Vang S, Corydon TJ, Borglum AD, etal (2005) Actin mutations in hypertrophic and dilated cardiomyopathy cause inefficient protein folding and perturbed filament formation. FEBS J 272: 2037–2049.
Varga K, Jurkuvenaite A, Wakefield J, etal (2004) Efficient intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J Biol Chem 279: 22578–22584.
Verbeke P, Fonager J, Clark BF, Rattan SI (2001) Heat shock response and ageing: mechanisms and applications. Cell Biol Int 25: 845–857.
Ward CL, Kopito RR (1994) Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J Biol Chem 269: 25710–25718.
Waters PJ (2003) How PAH gene mutations cause hyper-phenylalaninemia and why mechanism matters: insights from in vitro expression. Hum Mutat 21: 357–369.
Waters PJ, Parniak MA, Akerman BR, Jones AO, Scriver CR (1999) Missense mutations in the phenylalanine hydroxylase gene (PAH) can cause accelerated proteolytic turnover of PAH enzyme: a mechanism underlying phenylketonuria. J Inherit Metab Dis 22: 208–212.
Welsh MJ, Ramsey BN, Accurso F, Cutting GR (2001) Cystic fibrosis. In: Scriver CR, Beaudet al, Sly WS, Valle D, eds; Childs B, Kinzler KW, Vogelstein B, assoc, eds. The Metabolic and Molecular Bases of Inherited Disease, 8th edn. New York: McGraw-Hill, 5121–5188.
Westerheide SD, Morimoto RI (2005) Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem 280: 33097–33100.
Winyard PG, Moody CJ, Jacob C (2005) Oxidative activation of antioxidant defence. Trends Biochem Sci 30: 453–461.
Wiseman RL, Balch WE (2005) A new pharmacology — drugging stressed folding pathways. Trends Mol Med 11: 347–350.
Yin W, Kren BT, Steer CJ (2005) Site-specific base changes in the coding or promoter region of the human beta- and gamma-globin genes by single-stranded oligonucleotides. Biochem J 390: 253–261.
Zaccai G (2000) How soft is a protein? A protein dynamics force constant measured by neutron scattering. Science 288: 1604–1607.
Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ (2002) A mitochondrial specific stress response in mammalian cells. EMBO J 21: 4411–4419.
Zhou A, Stein PE, Huntington JA, Sivasothy P, Lomas DA, Carrell RW (2004) How small peptides block and reverse serpin polymerisation. J Mol Biol 342: 931–941.
Author information
Authors and Affiliations
Additional information
Communicating editor: Jean-Marie Saudubray
Competing interests: None declared
Rights and permissions
About this article
Cite this article
Gregersen, N. Protein misfolding disorders: Pathogenesis and intervention. J Inherit Metab Dis 29, 456–470 (2006). https://doi.org/10.1007/s10545-006-0301-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10545-006-0301-4