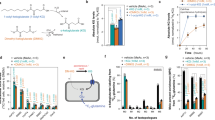
Summary
We investigated the presence of hydroxyacid–oxoacid transhydrogenase (HOT), which catalyses the cofactor-independent conversion of γ-hydroxybutyrate (GHB) to succinic semialdehyde coupled to reduction of 2-ketoglutarate (2-KG) to D-2-hydroxyglutarate (D-2-HG), in human liver extracts employing [2H6]GHB and 2-KG as substrates. We measured incorporation of 2H in D-[2H]2-HG using GC-MS analyses, providing evidence for HOT activity in humans. Kinetic characterization of HOT was undertaken in forward and reverse directions. We employed [2H6]GHB and [2H4]2-KG as cosubstrates in order to develop a HOT activity assay in cultured human fibroblasts derived from patients with D-2-hydroxyglutaric aciduria. HOT activity was quantified in this system by the measurement of D-[2H5]2-HG production. Fibroblasts derived from patients with D-2-hydroxyglutaric aciduria showed normal HOT activities. Our results provide the first demonstration and preliminary kinetic characterization of HOT activity in human tissues.
Similar content being viewed by others
References
Craigen WJ, Jakobs C, Sekul EA, et al (1994) D-2-Hydroxyglutaric aciduria in a neonate with seizures and CNS dysfunction. Pediatr Neurol 10: 49–53.
Gibson KM, Craigen W, Herman GE, et al (1993a) D-2-Hydroxyglutaric aciduria in a newborn with neurological abnormalities: a new neurometabolic disorder? J Inherit Metab Dis 16: 497–500.
Gibson KM, ten Brink HJ, Schor DSM, et al (1993b) Stable isotope dilution analysis of D- and L-2-hydroxyglutaric acid: application to the detection and prenatal diagnosis of D- and L-2-hydroxyglutaric acidemias. Pediatr Res 34: 277–280.
Jaeken J, Casaer P, de Cock P, et al (1984) Gamma-aminobutyric acid-transaminase deficiency: a newly recognized inborn error of neurotransmitter metabolism. Neuropediatrics 15: 165–169.
Kaufman EE, Nelson T, Fales MF, et al (1988a) Isolation and characterization of a hydroxyacid-oxoacid transhydrogenase from rat kidney mitochondria. J Biol Chem 263: 16872–16879.
Kaufman EE, Nelson T, Miller D, et al (1988b) Oxidation of gamma-hydroxybutyrate to succinic semialdehyde by a mitochondrial pyridine nucleotide-independent enzyme. J Neurochem 51: 1079–1084.
Loupatty FJ, Ruiter JPN, Ijlst L, et al (2004) Direct nonisotopic assay of 3-methylglutaconyl-CoA hydratase in cultured human skin fibroblasts to specifically identify patients with 3-methylglutaconic aciduria type 1. Clin Chem 50:1447–1450.
Nelson T, Kaufman EE (1994) Developmental time courses in the brain and kidney of two enzymes that oxidize γ-hydroxybutyrate. Dev Neurosci 16: 352–358.
Struys EA, Verhoeven NM, Roos B, et al (2003) Disease-related metabolites in culture medium of fibroblasts from patients with D-2-hydroxyglutaric aciduria, L-2-hydroxyglutaric aciduria, and combined d,l-2-hydroxyglutaric aciduria. Clin Chem 49: 1133–1138.
Struys EA, Salomons GS, Achouri Y, et al (2005) Mutations in the D-2-hydroxyglutarate dehydrogenase gene cause D-2-hydroxyglutaric aciduria. Am J Hum Genet 76: 358–360.
van der Knaap MS, Jakobs C, Hoffmann GF, et al (1999a) D-2-Hydroxyglutaric aciduria: biochemical marker or clinical disease entity? Ann Neurol 45: 111–119.
van der Knaap MS, Jakobs C, Hoffmann GF, et al (1999b) D-2-Hydroxyglutaric aciduria: further clinical dilineation. J Inherit Metab Dis 22: 404–413.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Struys, E.A., Verhoeven, N.M., Ten Brink, H.J. et al. Kinetic characterization of human hydroxyacid–oxoacid transhydrogenase: Relevance toD-2-hydroxyglutaric and γ-hydroxybutyric acidurias. J Inherit Metab Dis 28, 921–930 (2005). https://doi.org/10.1007/s10545-005-0114-x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10545-005-0114-x