Abstract
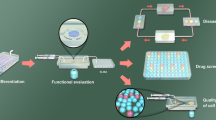
Transplantation of pancreatic islets is becoming a promising therapy for people with type I diabetes. In this study, we present a compact fluidic system that enables assessment of islet functionality ex vivo for efficient islet transplantation. The fluidic system includes a micromesh sheet-embedded chip. Islets can be loaded easily on the micromesh sheet and observed clearly by microscopy. Islets on the mesh sheet mainly remained in place during perfusion and did not get damaged by hydraulic pressure because of high porosity of the micromesh sheet. The fluidic system was assembled with a sample fraction chip of polydimethylsiloxane. The chip includes a channel and columns, both having surfaces that were super-hydrophilized so that solutions could flow smoothly within the chip by gravity. Using mouse pancreatic islets, a dynamic glucose-stimulated insulin secretion test was performed to examine the performance of the fluidic system. The system successfully analyzed levels and patterns of insulin secretion upon exposure of the islets to low- and high-glucose solutions in turns, thus demonstrating its capacity to assess islet functions more easily and cost-effectively.





Similar content being viewed by others
References
A.F. Adewola, D. Lee, T. Harvat, J. Mohammed, D.T. Eddington, J. Oberholzer, Y. Wang, Biomed. Microdevices 12(3), 409–417 (2010)
A. American Diabetes, Diabetes Care 32(Suppl 1), S62–S67 (2009)
S. Bauer, C. Wennberg Huldt, K.P. Kanebratt, I. Durieux, D. Gunne, S. Andersson, L. Ewart, W.G. Haynes, I. Maschmeyer, A. Winter, C. Ammala, U. Marx, T.B. Andersson, Sci. Rep. 7(1), 14620 (2017)
M. Brissova, M.J. Fowler, W.E. Nicholson, A. Chu, B. Hirshberg, D.M. Harlan, A.C. Powers, J. Histochem. Cytochem. 53(9), 1087–1097 (2005)
O. Cabrera, D.M. Berman, N.S. Kenyon, C. Ricordi, P.O. Berggren, A. Caicedo, Proc. Natl. Acad. Sci. U. S. A. 103(7), 2334–2339 (2006)
O. Cabrera, M.C. Jacques-Silva, D.M. Berman, A. Fachado, F. Echeverri, R. Poo, A. Khan, N.S. Kenyon, C. Ricordi, P.O. Berggren, A. Caicedo, Cell Transplant. 16(10), 1039–1048 (2008)
D. Chen, W. Du, Y. Liu, W. Liu, A. Kuznetsov, F.E. Mendez, L.H. Philipson, R.F. Ismagilov, Proc. Natl. Acad. Sci. U. S. A. 105(44), 16843–16848 (2008)
S. Chon, J.F. Gautier, Diabetes Metab. J. 40(2), 99–114 (2016)
J.F. Dishinger, R.T. Kennedy, Anal. Chem. 79(3), 947–954 (2007)
K. Inoue, M. Miyamoto, J. Hepato-Biliary-Pancreat. Surg. 7(2), 163–177 (2000)
International Diabetes Federation, 8th edn. (2017)
S. Konagaya, H. Iwata, Biochim. Biophys. Acta 1860(9), 2008–2016 (2016)
S. Konagaya and H. Iwata, Scientific Reports 9 (1) (2019)
G. Lenguito, D. Chaimov, J.R. Weitz, R. Rodriguez-Diaz, S.A. Rawal, A. Tamayo-Garcia, A. Caicedo, C.L. Stabler, P. Buchwald, A. Agarwal, Lab Chip 17(5), 772–781 (2017)
D.M. Maahs, N.A. West, J.M. Lawrence, E.J. Mayer-Davis, Endocrinol. Metab. Clin. N. Am. 39(3), 481–497 (2010)
J.S. Mohammed, Y. Wang, T.A. Harvat, J. Oberholzer, D.T. Eddington, Lab Chip 9(1), 97–106 (2009)
M. Nourmohammadzadeh, J.F. Lo, M. Bochenek, J.E. Mendoza-Elias, Q. Wang, Z. Li, L. Zeng, M. Qi, D.T. Eddington, J. Oberholzer, Y. Wang, Anal. Chem. 85(23), 11240–11249 (2013)
M. Nourmohammadzadeh, Y. Xing, J.W. Lee, M.A. Bochenek, J.E. Mendoza-Elias, J.J. McGarrigle, E. Marchese, Y. Chun-Chieh, D.T. Eddington, J. Oberholzer, Y. Wang, Lab Chip 16(8), 1466–1472 (2016)
A. Pileggi, C. Ricordi, N. S. Kenyon, T. Froud, D. A. Baidal, A. Kahn, G. Selvaggi and R. Alejandro, Clin Transpl, 177–204 (2004)
J.V. Rocheleau, G.M. Walker, W.S. Head, O.P. McGuinness, D.W. Piston, Proc. Natl. Acad. Sci. U. S. A. 101(35), 12899–12903 (2004)
M.G. Roper, J.G. Shackman, G.M. Dahlgren, R.T. Kennedy, Anal. Chem. 75(18), 4711–4717 (2003)
K. Saito, N. Iwama, T. Takahashi, Tohoku J. Exp. Med. 124(2), 177–186 (1978)
V. Scattolini, C. Luni, A. Zambon, S. Galvanin, O. Gagliano, C.D. Ciubotaru, A. Avogaro, F. Mammano, N. Elvassore, G.P. Fadini, Diabetes Ther 7(4), 679–693 (2016)
J.G. Shackman, G.M. Dahlgren, J.L. Peters, R.T. Kennedy, Lab Chip 5(1), 56–63 (2005)
I.R. Sweet, M. Gilbert, S. Scott, I. Todorov, R. Jensen, I. Nair, I. Al-Abdullah, J. Rawson, F. Kandeel, K. Ferreri, Am. J. Transplant. 8(1), 183–192 (2008)
L. Tao, R.T. Kennedy, Anal. Chem. 68(22), 3899–3906 (1996)
L. Tao, C.A. Aspinwall, R.T. Kennedy, Electrophoresis 19(3), 403–408 (1998)
D. Yabe, Y. Seino, Prog. Biophys. Mol. Biol. 107(2), 248–256 (2011)
Acknowledgments
This work was supported in part by a grant-in-aid from the Compass to Healthy Life Research Complex Program, RIKEN (Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Fig. 1
Designs of the micromesh chip. A partial perspective view of the micromesh chip (top panel) and a blueprint of the chip (bottom panel) are shown. (PPTX 92 kb)
Supplemental Fig. 2
Conditions of fluid flow in the micromesh ship and designs of the Y-connector. (A) A schematic diagram of a fluidic system to confirm fluid flow in the micromesh chip. PBS(−) or 0.01 w/v% trypan blue solution was introduced into the chip at 50 μL/min by syringe pumps. (B) Picture of the Y-connector of PDMS. (C) PBS(−) was flowed by a syringe pump (the left figure) and then the solution was switched to a trypan blue solution (the right figure). Yellow arrows indicate the direction of fluid flow. (PPTX 954 kb)
Supplemental Fig. 3
Fluid flow in the fraction chip with 12 columns. (A) Assignation of numbers to each column. (B) Images show how solutions flow within the chip up to 12 columns. Solutions were indicated in blue. First of all, introduced solutions go to the bottom column in sheet a (column no. 1) by gravity. At this time, these solutions cannot flow in the sheet b because the aperture leading to the channel in sheet b is higher in place than the point of the tube. After 6 columns were filled with solutions, the surface of the solutions can close to the aperture and then flow into the channel in sheet b. Running through the aperture, solutions can be stored from the bottom column (column no. 7) to the upper column (column no. 12) in sheet b by gravity. Introducing solutions was terminated by stopping pumps manually when all 12 columns were filled with solutions. (C) . Designs of the fraction chip. Blueprints of the sheet a (left panel) and sheet b (right panel) are shown. (PPTX 238 kb)
Supplemental Fig. 4
A method to recover solutions from a fraction chip without spills. By removing solutions of an inner channel using a Pasteur pipette, solutions can be recovered without spills during cutting the chip. (PPTX 57 kb)
Supplemental Fig. 5
The 6-column fraction chip. (A) A schematic diagram showing the method to fabricate Sheet a of the 6-column fraction chip. The PDMS surface of an inner channel and columns was super-hydrophilized so that solutions flow smoothly within the chip by gravity. The inside surface was coated with Parylene and then treated with energetic air-plasma using a plasma cleaner. (B) For a Sheet b, a flat 1 mm-thick PDMS sheet was, in turns, coated with Parylene and silica-based materials, which are Selface coat WG-R1and NZ-550, and then treated with energetic plasma to increase hydrophilicity. (C) The Sheet a and b were combined. A 2-mm diameter aperture was punched in the Sheet a for the air-outlet. A tube of a micromesh chip was connected with the inlet of the fraction chip. (D) Perfusion test for the chip. Colored solutions were run into the fraction chip to observe the flow and mixing of the solutions in the chip. Following yellow solutions were run into the chip, blue solutions were run into it at 50 μL/min. A yellow solution successfully flowed in the chip, and then a blue solution also flowed smoothly. This collection chip was able to chronologically collect solutions without mixing them between columns. (PPTX 2617 kb)
Rights and permissions
About this article
Cite this article
Hori, T., Yamane, K., Anazawa, T. et al. Compact fluidic system for functional assessment of pancreatic islets. Biomed Microdevices 21, 91 (2019). https://doi.org/10.1007/s10544-019-0443-4
Published:
DOI: https://doi.org/10.1007/s10544-019-0443-4