Abstract
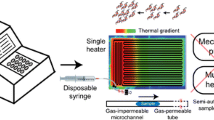
Polymerase Chain Reaction (PCR) is used to amplify a specific segment of DNA through a thermal cycling protocol. The PCR industry is shifting its focus away from macro-scale systems and towards micro-scale devices because: micro-scale sample sizes require less blood from patients, total reaction times are on the order of minutes opposed to hours, and there are cost advantages as many microfluidic devices are manufactured from inexpensive polymers. Some of the fastest PCR devices use continuous flow, but they have all been built of silicon or glass to allow sufficient heat transfer. This article presents a disposable polycarbonate (PC) device that is capable of achieving real-time, continuous flow PCR in a completely disposable polymer device in less than 13 minutes by thermally cycling the sample through an established temperature gradient in a serpentine channel. The desired temperature gradient was determined through simulations and validated by experiments which showed that PCR was achieved. Practical demonstration included amplification of foot-and-mouth disease virus (FMDV) derived cDNA.











Similar content being viewed by others
References
S. R. Beard, et al., Microwell Array PCR Chip for Study of Genetically Engineered Mouse Stem Cells, presented at the MicroTAS, Seattle, Washington, (2011)
Q. Q. Cao, et al., Plastic microfluidic chip for continuous flow polymerase chain reaction: Simulations and experiments, Biotechnology Journal, (2010)
J. Chen et al., Electrokinetically synchronized polymerase chain reaction microchip fabricated in polycarbonate. Anal. Chem. 77, 658–666 (2005)
L. Chen et al., Ultrasensitive PCR and real-time detection from human genomic samples using a bidirectional flow microreactor. Anal. Chem. 79, 9185–9190 (2007)
J. Y. Cheng et al., Performing microchannel temperature cycling reactions using reciprocating reagent shuttling along a radial temperature gradient. Analyst 130, 931–940 (2005)
H. Cheun et al., Rapid and effective detection of anthrax spores in soil by PCR. J. Appl. Microbiol. 95, 728–733 (2003)
J. Chiou et al., A closed-cycle capillary polymerase chain reaction machine. Anal. Chem. 73, 2018–2021 (2001)
N. Crews, C. Wittwer, B. Gale, Continuous-flow thermal gradient PCR. Biomed. Microdevices 10(2), 187–195 (2008)
K. D. Dorfman et al., Contamination-free continuous flow microfluidic polymerase chain reaction for quantitative and clinical applications. Anal. Chem. 77, 3700–3704 (2005)
H. A. Erlich, PCR technology: principles and applications for DNA amplification (W.H. Freeman and Company, New York, 1992)
A. Hühmer, J. Landers, Noncontact infrared-mediated thermocycling for effective polymerase chain reaction amplification of DNA in nanoliter volumes. Anal. Chem. 72, 5507–5512 (2000)
M. Kanai, et al., A novel contamination free PCR well Array device for clinical applications, presented at the MicroTAS 2011, Seattle, Washington, (2011)
M. U. Kopp et al., Chemical amplification: continuous-flow PCR on a chip. Science 280, 1046 (1998)
T. M. H. Lee et al., Microfabricated PCR-electrochemical device for simultaneous DNA amplification and detection. Lab Chip 3, 100–105 (2003)
S. Li et al., A continuous-flow polymerase chain reaction microchip with regional velocity control. J. Microelectromech. Syst. 15, 223–236 (2006)
J. A. Lounsbury, et al., A multi-chamber PMMA Microdevice for simultaneous amplification of up to Sevel individual samples using infrared-mediated PCR, presented at the MicroTAS 2011, Seattle, Washington, (2011)
S. I. Makino et al., Detection of anthrax spores from the air by real-time PCR. Lett. Appl. Microbiol. 33, 237–240 (2001)
R. Oda et al., Infrared-mediated thermocycling for ultrafast polymerase chain reaction amplification of DNA. Anal. Chem. 70, 4361–4368 (1998)
R. L. Orozco, Computational heat transfer study of thermal gradient continuous flow polymerase chain reaction devices (University of Utah, Department of Mechanical Engineering, 2009)
Y. Ouyang, et al., Development of disposable Multichambered microchip PCR via non-contact IR mediated thermal control, presented at the MicroTAS 2011, Seattle, Washington, (2011)
N. Park et al., Cylindrical compact thermal-cycling device for continuous-flow polymerase chain reaction. Anal. Chem. 75, 6029–6033 (2003)
S. D. J. Pena, R. Chakraborty, Paternity testing in the DNA era. Trends Genet. 10, 204–209 (1994)
I. Schneega et al., Miniaturized flow-through PCR with different template types in a silicon chip thermocycler. Lab Chip 1, 42–49 (2001)
M. A. Shoffner et al., Chip PCR. I. Surface passivation of microfabricated silicon-glass chips for PCR. Nucleic Acids Res. 24, 375 (1996)
S. Sundberg, C. Wittwer, C. Gao, B. Gale, Spinning disk platform for microfluidic digital polymerase chain reaction. Anal. Chem. 82, 1546–1550 (2010)
C. T. Wittwer and M. G. Herrmann, Rapid thermal cycling and PCR kinetics, PCR applications: protocols for functional genomics (1999), p. 211
C. Wittwer et al., Automated polymerase chain reaction in capillary tubes with hot air. Nucleic Acids Res. 17, 4353 (1989)
Acknowledgements
The authors would like to acknowledge Carl Wittwer and his laboratory for help with PCR testing. We also acknowledge Indian Immunologicals Ltd. Hyderabad, India for partial funding towards testing of the microfluidic PCR chip and FMDV viral culture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was partially funded by Indian Immunological Ltd., Hyderabad, India.
Conflict of interest
The authors declare that they have no conflict of interest.
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Ragsdale, V., Li, H., Sant, H. et al. A disposable, continuous-flow polymerase chain reaction device: design, fabrication and evaluation. Biomed Microdevices 18, 62 (2016). https://doi.org/10.1007/s10544-016-0091-x
Published:
DOI: https://doi.org/10.1007/s10544-016-0091-x