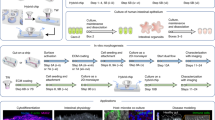
Abstract
Physiological and morphological properties of the human intestine cannot be accurately mimicked in conventional culture devices such as well plates and petri dishes where intestinal epithelial cells form a monolayer with loose contacts among cells. Here, we report a novel microfluidic cell culture device (μFCCD) that can be used to culture cells as a human intestinal model. This device enables intestinal epithelial cells (Caco-2) to grow three-dimensionally on a porous membrane coated with fibronectin between two polydimethylsiloxane (PDMS) layers. Within 3 days, Caco-2 cells cultured in the μFCCD formed villi- and crypt-like structures with small intercellular spaces, while individual cells were tightly connected to one another through the expression of the tight junction protein occludin, and were covered with a secreted mucin, MUC-2. Caco-2 cells cultured in the μFCCD for 3 days were less susceptible to bacterial attack than those cultured in transwell plates for 21 days. μFCCD-cultured Caco-2 cells also displayed physiologically relevant absorption and paracellular transport properties. These results suggest that our intestinal model more accurately mimics the morphological and physiological properties of the intestine in vivo than the conventional transwell culture model.





Similar content being viewed by others
References
M. Andrianifahanana, N. Moniaux, S.K. Batra, Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim. Biophys. Acta 1765(2), 189–222 (2006)
P. Artursson, K. Palm, K. Luthman, Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 46(1–3), 27–43 (2001)
M.D. Basson, Paradigms for mechanical signal transduction in the intestinal epithelium. Category: molecular, cell, and developmental biology. Digestion 68(4), 217–225 (2003)
J.M. Biazik, K.A. Jahn, Y. Su, Y.N. Wu, F. Braet, Unlocking the ultrastructure of colorectal cancer cells in vitro using selective staining. World J. Gastroenterol. 16(22), 2743–2753 (2010)
X.D. Bu, N. Li, X.Q. Tian, P.L. Huang, Caco-2 and LS174T cell lines provide different models for studying mucin expression in colon cancer. Tissue Cell 43(201), 201–206 (2011)
J.C. Byrd, R.S. Bresalier, Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 23(1–2), 77–99 (2004)
M. Dickson, J.P. Gagnon, Key factors in the rising cost of new drug discovery and development. Nat. Rev. Drug Discov. 3(5), 417–429 (2004)
L.C. Duffy, Interactions mediating bacterial translocation in the immature intestine. J. Nutr. 130(2S Suppl), 432S–436S (2000)
M.B. Esch, J.H. Sung, J. Yang, C. Yu, J. Yu, J.C. March, M.L. Shuler, On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic ‘body-on-a-chip’ devices. Biomed. Microdevices 14(5), 895–906 (2012)
G.J. Feldman, J.M. Mullin, M.P. Ryan, Occludin: structure, function and regulation. Adv. Drug Deliv. Rev. 57(6), 883–917 (2005)
B.E. Goodman, Insights into digestion and absorption of major nutrients in humans. Adv. Physiol. Educ. 34(20), 44–53 (2010)
B.M. Gumbiner, Structure, biochemistry, and assembly of epithelial tight junctions. Am. J. Physiol. 253(6 Pt 1), C749–C758 (1987)
B.M. Gumbiner, Breaking through the tight junction barrier. J. Cell Biol. 123(6 Pt 2), 1631–1633 (1993)
P. Guo, A.M. Weinstein, S. Weinbaum, A hydrodynamic mechanosensory hypothesis for brush border microvilli. Am J Physiol Renal Physiol. 279(4), F698–F712 (2000)
J. Hansen, L. Andrew, R. Ruedy, M. Sato, Potential climate impact of mount pinatubo eruption. Geophys. Res. Lett. 19(2), 215–218 (1992)
D.H. Huh, Y.S. Torisawa, G.A. Hamilton, H.J. Kim, D.E. Ingber, Microengineered physiological biomimicry: organs-on-chips. Lab Chip 12(12), 2156–2164 (2012)
Y. Imura, Y. Asano, K. Sato, E. Yoshimura, A microfluidic system to evaluate intestinal absorption. Anal. Sci. 25, 1403–1407 (2009)
T. Ishikawa, T. Sato, G. Mohit, Y. Imai, T. Yamaguchi, Transport phenomena of microbial flora in the small intestine with peristalsis. J. Theor. Biol. 279(1), 63–73 (2011)
K. Izumikawa, Y. Hirakata, T. Yamaguchi, H. Takemura, S. Maesaki, K. Tomono, S. Igimi, M. Kaku, Y. Yamada, S. Kohno, S. Kamihira, Escherichia coli O157 interactions with human intestinal Caco-2 cells and the influence of fosfomycin. J. Antimicrob. Chemother. 42(3), 341–347 (1998)
Y. Jin, Y. Takegahara, Y. Sugawara, T. Matsumura, Y. Fujinaga, Disruption of the epithelial barrier by botulinum haemagglutinin (HA) proteins - differences in cell tropism and the mechanism of action between HA proteins of types A or B, and HA proteins of type C. Microbiology 155(Pt 1), 35–45 (2009)
J.A. Kiernan, Histological and histochemical methods: theory and practice (Butterworth Heinemann, Oxford, 1999). x, 502 p
H.J. Kim, D.E. Ingber, Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol. 5(9), 1130–1140 (2013)
H.J. Kim, D. Huh, G. Hamilton, D.E. Ingber, Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12(12), 2165–2174 (2012)
S.H. Kim, J.W. Lee, I. Choi, Y.C. Kim, J.B. Lee, J.H. Sung, A microfluidic device with 3-d hydrogel villi scaffold to simulate intestinal absorption. J. Nanosci. Nanotechnol. 13(11), 7220–7228 (2013)
S.H. Kim, M. Chi, B. Yi, S.H. Kim, S. Oh, Y. Kim, S. Park, J.H. Sung, Three-dimensional intestinal villi epithelium enhances protection of human intestinal cells from bacterial infection by inducing mucin expression. Integr. Biol. 6(12), 1122–1131 (2014)
R.G. Lentle, P.W.M. Janssen, Physical characteristics of digesta and their influence on flow and mixing in the mammalian intestine. J. Comp. Physiol. B. 178(6), 673–690 (2008)
F. Leonard, E.M. Collnot, C.M. Lehr, A three-dimensional coculture of enterocytes, monocytes and dendritic cells to model inflamed intestinal mucosa in vitro. Mol. Pharm. 7, 2103–2119 (2010)
N. Li, D. Wang, Z. Sui, X. Qi, L. Ji, X. Wang, L. Yang, Development of an improved three-dimensional in vitro intestinal mucosa model for drug absorption evaluation. Tissue Eng. Part C Methods 19(9), 708–719 (2013)
O. Lieleg, I. Vladescu, K. Ribbeck, Characterization of particle translocation through mucinhydrogels. Biophys. J. 98(9), 1782–1789 (2010)
K.M. McCarthy, I.B. Skare, M.C. Stankewich, M. Furuse, S. Tsukita, R.A. Rogers, R.D. Lynch, E.E. Schneeberger, Occludin is a functional component of the tight junction. J. Cell Sci. 109, 2287–2298 (1996)
I. Meyvantsson, D.J. Beebe, Cell culture models in microfluidic systems. Annu. Rev. Anal. Chem. 1, 423–449 (2008)
K.W. Oh, L. Lee, B. Ahn, E.P. Furlani, Design of pressure-driven microfluidic networks using electric circuit analogy. Lab Chip 12(3), 515–545 (2012)
J. Olesen, L. Edvinsson, Basic mechanisms of headache, Amsterdam; NY, USA: Elsevier; Sole distributors for the USA and Canada, Elsevier Science Pub. Co. xxvi, 492 p (1988)
S.P. Olesen, P.F. Davies, D.E. Clapham, Muscarinic-activated K+ current in bovine endothelial cells. Circ. Res. 62(2), 1059–1064 (1988b)
E. Panteris, P. Apostolakos, B. Galatis, Microtubule organization, mesophyll cell morphogenesis, and intercellular space formation in Adiantum capillus veneris leaflets. Protoplasma 172(2–4), 97–110 (1993)
S. Park, H.J. Chun, J.S. Jang, B. Keum, Y.S. Seo, Y.S. Kim, Y.T. Jeen, H.S. Lee, S.H. Um, C.D. Kim, H.S. Ryu, C.S. Uhm, S.J. Lee, Is intercellular space different among layers in normal esophageal mucosa? An electron microscopic study. Dig. Dis. Sci. 56(12), 3492–3497 (2011)
B.M.H. Schneeberger, Die Musikerfamilie Fürstenau: Untersuchungen zu Leben und Werk (Lit, Münster, 1992)
J.P. Schouten, Revolution of the mystics: on the social aspects of Vīraśaivism (Kok Pharos Pub. House, Kampen, 1991). xiii, 331 p
J.M. Staddon, L.L. Rubin, Cell adhesion, cell junctions and the blood-brain barrier. Curr. Opin. Neurobiol. 6(5), 622–627 (1996)
T.M. Straub, J.R. Hutchison, R.A. Bartholomew, C.O. Valdez, N.B. Valentine, A. Dohnalkova, R.M. Ozanich, C.J. Bruckner-Lea, Defining cell culture conditions to improve human norovirus infectivity assays. Water Sci. Technol. 67(4), 863–868 (2013)
J.H. Sung, J. Yu, D. Luo, M.L. Shuler, J.C. March, Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip 11(3), 389–392 (2011)
J.H. Sung, M.B. Esch, J.M. Prot, C.J. Long, A. Smith, J.J. Hickman, M.L. Shuler, Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab Chip 13(7), 1201–1212 (2013)
F.J. Van Asten, H.G. Hendriks, J.F. Koninkx, B.A. Van der Zeijst, W. Gaastra, Inactivation of the flagellin gene of Salmonella enterica serotype Enteritidis strongly reduces invasion into differentiated Caco-2 cells. FEMS Microbiol. Lett. 185(2), 175–179 (2000)
R.B. van Breemen, Y. Li, Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 1, 175–185 (2005)
P. Zanassi, M. Paolillo, A. Feliciello, E.V. Avvedimento, V. Gallo, S. Schinelli, cAMP-dependent protein kinase induces cAMP-response element-binding protein phosphorylation via an intracellular calcium release/ERK-dependent pathway in striatal neurons. J. Biol. Chem. 276(15), 11487–11495 (2001)
Acknowledgments
This work was supported by a grant from the Korea Science and Engineering Foundation (KOSEF) funded by the Korea government (MOST) (#2012-0001138), and by a grant from the Public Welfare & Safety Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (#2012R1A2A2A01012221 and #2012-0006522). JHS and BL acknowledges support from Hongik University Research Fund.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chi, M., Yi, B., Oh, S. et al. A microfluidic cell culture device (μFCCD) to culture epithelial cells with physiological and morphological properties that mimic those of the human intestine. Biomed Microdevices 17, 58 (2015). https://doi.org/10.1007/s10544-015-9966-5
Published:
DOI: https://doi.org/10.1007/s10544-015-9966-5